
10 Facts From the UK Government Pfizer Vaccine Guidance That Promote “Vaccine Hesitancy”
Official government guidance has been released in the United Kingdom to assist healthcare professionals in administering the Pfizer/BioNTech vaccine BNT162b2. While the UK government goes to war against supposed misinformation, the official narrative is clearly based on very little to no supporting data from incomplete clinical trials. This article examines the document “Reg 174 Information for UK Healthcare Professionals” and narratives being pushed in the mainstream media that directly contradict that document.
by Johnny Vedmore, Unlimited Hangout
December 30, 2020
Healthcare professionals globally have begun the controversial campaign to vaccinate large swathes of their respective populations with various experimental medical products. The vanguard of the mainstream pro-vax extremists have been busy enacting mass censorship tactics and committing blatant acts of digital book burning on a scale never before seen in the internet era. So-called “trusted sources” have become indistinguishable from the state-run media apparatus of your bog-standard dictatorship with the usual MSM outlets working non-stop to skew any information that threatens their hyper-aggressive official narrative. Throughout 2020, our basic civil liberties have been quickly stripped away by countless unelected officials from a wide array of unaccountable global power structures, all of them connected to a small group of elites who are sitting aloft the COVID-19 money train and using the heavily exaggerated epidemic to achieve their own long term goals.
Any useful data, scientific paper, or other credible research contradicting the official narrative is being purposely hidden from view. Too many uncomfortable, yet ultimately necessary, questions for vaccine companies such as Moderna, AstraZeneca, Pfizer, and their many collaborators, are being heavily censored by those pushing their own various COVID-related agendas. The promised “war on truth” is in full swing throughout all nations globally and their respective state media machines are nearly all towing their official government lines. Mainstream talk shows and podcasts worldwide are also in lockstep, and have often been caught publicly guilt-tripping their easily swayed audiences to help push them deeper into queues for mass medical trials for vaccines and other products that lack research studies on their long term effects. This inconvenient lack of completed research will not stop the money men from pumping this milky white liquid into the arms of hundreds of millions of people worldwide.
At this point in the process, the medical professionals who are administering these heavily rushed vaccines are being given the opportunity to defer responsibility and accountability for their actions to the government’s vaccine-related guidance. As the Stanley Milgram experiments have proven, when the option to defer responsibility is present, then roughly 65% of participants will follow the orders they have received regardless of the risk to their subjects. In 1974, Stanley Milgram detailed the behaviour of his participants in his famous study and suggested that people have two basic states of behaviour when they are in a social situation: “The autonomous state”, where people direct their own actions and ultimately take responsibility for the results of those actions and “the agentic state”, where people allow others to direct their actions and then pass off the responsibility for the consequences to the person giving orders, in essence acting as agents of another person’s will.
The majority of the people who are injecting these experimental drugs into their trusting patients are not likely to question the official guidance, as the overwhelming majority will often simply be in an agentic state. Thus, it should be in the best interest of anyone thinking of receiving a mRNA vaccine to first study the guidance offered by the various government sources. And, when one does study the official guidance given to healthcare professionals, one will find many different glaring contradictions and shocking admissions.
While all official bodies are attacking any inconvenient fact as misinformation, they are all busy defrauding the global population with their own misinformation campaigns that surely would have inspired awe in the likes of Joseph Stalin. So, let’s study their own words and examine the NHS guidance given to the medical professionals in the UK for the administration of the recently approved Pfizer-BioNTech vaccine.
An Introduction to Reg 174 Information for UK Healthcare Professionals (#1-4)
The short ten page official guidance being given to UK healthcare professionals contains many interesting admissions. In fact, the document, released in early December 2020 to accompany the vaccine rollout, appears to advise healthcare practitioners not to risk giving the experimental injection to the majority of the people who are due to receive the vaccine, particularly “prioritized” populations. Those in charge are pushing to vaccinate as much of the population as possible, before any critical public questions can be asked and answered, a situation that has left the safety and ethics of the vaccination campaign questionable at best and inhumane at worst.
In going through the Reg 174 document, it becomes very clear that there are many issues and recommendations that are being hidden from the general public. Here are ten of the most notable causes for concern contained within the official UK guidance document.
1. This medicinal product does not have UK marketing authorisation but has been given authorisation only for temporary supply
The authorisation to produce and supply this experimental vaccine in the UK was given by the UK Department of Health and Social Care, led by Matt Hancock – the UK Secretary of Health, and also by the Medicines & Healthcare products Regulatory Agency (MHRA). While the MHRA is part funded by the Department of Health and Social Care for the regulation of medical devices, the costs of medicine regulations are met through fees paid by the pharmaceutical industry. The agency’s financial reliance on Big Pharma has led to suggestions by some Members of the UK Parliament that the MHRA is not actually independent. Being in associated roles at the MHRA since 1985, June Raine was officially appointed as CEO in September 2019 and had previously been the Director of Vigilance and Risk Management in the Medicines Division.
2. The official Phase III safety trials will not be completed until 2023
Section 1 of the medical guidance clearly states that this vaccine guidance refers specifically to the “Pfizer/BioNTech COVID-19 mRNA Vaccine BNT162b2 concentrate for solution for injection.” On 2 December 2020, the MHRA became the first medicines regulator in history to approve an mRNA vaccine for human use, granting emergency authorisation for BioNTech and Pfizer’s BNT162b2 COVID-19 vaccine for widespread use only a week after its first Phase III eight-week trial had finished. However, the Phase III trials for BNT162b2 will not actually be fully completed until January 2023 meaning that, if you’re ready to take the vaccine now, then you should be informed that the safety trials for these experimental vaccines have at least two more years before the results are in. Regardless of that fact, Raine told reporters “no corners have been cut in approving it” and that “the benefits outweigh any risk”.
3. Will you be truly “protected” from COVID-19?
The official guidance clearly states that individuals may not be protected until at least 7 days after their second dose of the vaccine. This fact has again been ignored by various reckless pro-vax media campaigns where powerful elites such as Tony Blair have contradicted this specific recommendation, suggesting recently in an interview that people should only be given a single dose of any vaccine. Mr Blair told BBC Radio 4’s Today programme that “Does the first dose give you substantial immunity, and by that I mean over 50 percent effectiveness? If it does, there is a very strong case for not, as it were, holding back doses of the vaccine.” Blair, writing in the Independent, stated that the current vaccination strategy needed to be “altered and radically accelerated”. In responding to Blair’s call for radical acceleration, Professor Wendy Barclay, chair of virology at Imperial College London and member of the UK government’s NERVTAG, said: “I think that the issue with [Mr Blair’s suggestion] is that the vaccine is on the basis of being given in two doses, and the efficacy is on that basis.” Barclay went on to point out that “To change at that point, one would have to see a lot more analysis coming out from perhaps the clinical trial data.”
It is very important to pay attention to the wording of Reg 174 because the Pfizer vaccine purportedly boosts the immune system, rather than stopping the transmission of the virus. This would suggest that you will not be fully “protected” from COVID-19 and that you will still be able to catch the virus and could still suffer complications. The official guidance also states that “Immunocompromised persons, including individuals receiving immunosuppressant therapy, may have a diminished immune response to the vaccine,” with the guidance admitting “No data are available about concomitant use of Immunosuppressants.”
Reg 174 goes on to make this most pertinent of points when it states: “As with any vaccine, vaccination with COVID-19 mRNA Vaccine BNT162b2 may not protect all vaccine recipients.” The guidance also states clearly that “administration of COVID-19 mRNA Vaccine BNT162b2 should be postponed in individuals suffering from acute severe febrile illness and that individuals receiving anticoagulant therapy or those with a bleeding disorder that would contraindicate intramuscular injection, should not be given the vaccine unless the potential benefit clearly outweighs the risk.”
4. The complicated multistage dilution and thawing process of the vaccine vials opens the major possibility of human error
In investigating the official instructions for the vaccine’s administration, we can clearly see that there are plenty of opportunities for potential human error. Section 2 of this document describes the distributed vaccine as coming in “a multidose vial and must be diluted before use.” Confirming that each vial contains 0.45 ml (which equates to 5 doses of 30 micrograms) of BNT162b2 RNA embedded in lipid nanoparticles. The delicate preparation process will be repeated 100s of millions of times globally and the multidose vial will be stored frozen and must be thawed prior to dilution. The guidance describes the process for preparing the frozen vials stating that they should be transferred to temperatures of between 2 °C to 8 °C to thaw or, alternatively, the frozen vials may also be thawed for 30 minutes at temperatures up to 25 °C for immediate use. Once thawed, the undiluted vaccine can be stored for up to 5 days at 2 °C to 8 °C, and up to 2 hours at temperatures up to 25 °C. The thawed vial must then come to room temperature and be gently inverted 10 times prior to dilution.
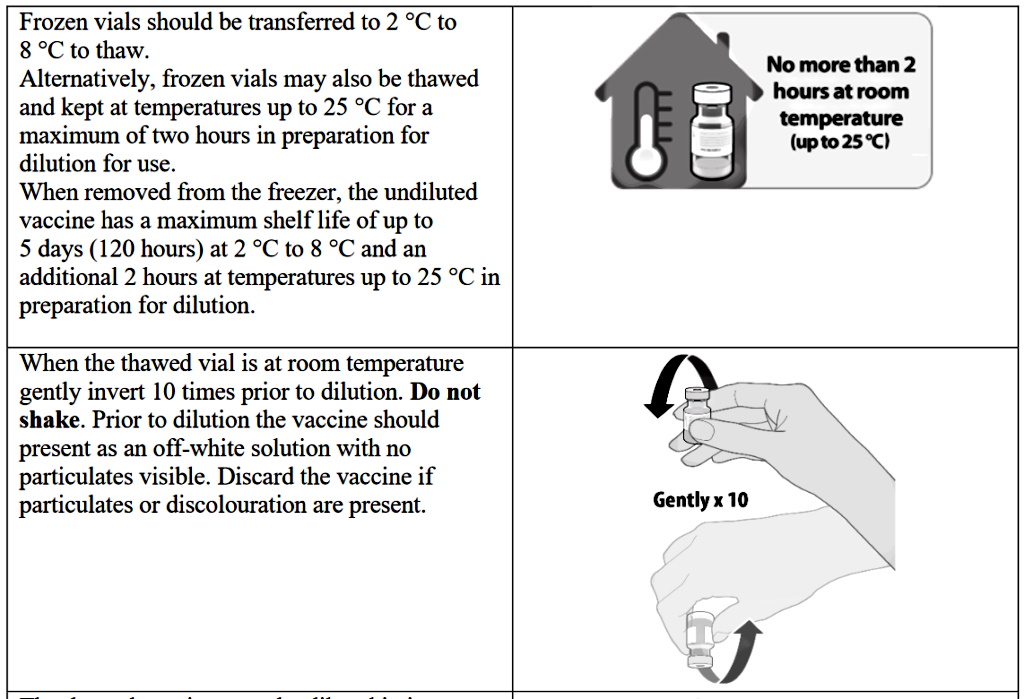
The complicated thawing and dilution process will obviously leave room for individual error. Healthcare practitioners are also warned not to shake the vials and instead to gently turn them 10 times. Prior to dilution, the vaccine should present as an off-white solution with no particulates visible. The guidance states that you must discard the vaccine if particulates or discolouration are present. The thawed vaccine must be diluted in its original vial with 1.8 mL sodium chloride 9 mg/mL (0.9%) solution for injection, using a 21 gauge or narrower needle and aseptic techniques and this complex, multistage process isn’t completed there.
The healthcare professional should then equalise vial pressure before removing the needle from the vial by withdrawing 1.8 mL of air into the empty diluent syringe. Then they should gently invert the diluted solution 10 times, again being careful not to shake the solution. The official guidance continues: “The diluted vials should be marked with the dilution date and time and stored between 2 °C to 25 °C. After dilution, the vial contains 5 doses of 0.3 mL.” The healthcare professionals are then told to “withdraw the required 0.3 mL dose of diluted vaccine using a sterile needle and syringe and discard any unused vaccine within 6 hours after dilution.”
The instructions must be followed precisely to safely administer the mRNA vaccine; there are no data available on potential consequences for the vaccine recipient if anything goes wrong during this tedious and complex multistage process. On 19 December 2020, video emerged of an official drive-thru vaccination hub which had begun operating out of a car park of Hyde Leisure Centre in Greater Manchester. The video in question, shared by No Comment TV on YouTube, shows people being vaccinated outdoors at Hyde Leisure Centre by gloveless staff and in less than sterile conditions. In an article in the Manchester Evening News four days prior to the videos release the local news site stated that “The first batch of the Pfizer/BioNTech vaccine arrives in the borough on Tuesday, with vaccinations starting at Hyde Leisure Centre on Wednesday, December 15.”
No Data Available (#5-10)
When reading Reg 174, you will soon notice a recurring theme throughout the document. The guidance clearly states on multiple occasions that there are no data available concerning some of the most important questions surrounding the mRNA vaccine. As previously noted, the actual Phase III section of the safety trials will not be completed until January 2023, meaning that two years of trials are still to be run before the vaccine can be confirmed as safe, effective and ethical.
5. The safety and efficacy of COVID-19 mRNA Vaccine BNT162b2 in children under 16 years of age have not yet been established
Although the guidance states that the safety and efficacy of the COVID-19 vaccine has not been established in children, it doesn’t mean that children have not been included within the studies. In fact, in the official Pfizer study entitled “Protocol C4591001”, one of the two main study groups included children as young as 12 years old. The inclusion of children in trials but not the guidance raises the important question, why were children included in the trial? If the vaccine is not to be given to those under the age of 16 years old, then why include children as young as 12 in the trials for an experimental vaccine technology never before authorised for use in humans?
The mainstream media, instead of raising concerns about the involvement of children in the Pfizer clinical trials, have been fully supportive of the move to test experimental pharmaceuticals on minors. CNN reported on children as young as 12 being involved in trials in an October 2020 article entitled “This 12-year-old is happy to be testing a Covid-19 vaccine” while Microsoft News recently announced that “China begins Covid test trials on children as young as age three.”
6. No data are available on the use of COVID-19 mRNA Vaccine BNT162b2 in persons that have previously received a full or partial vaccine series with another COVID-19 vaccine
We are currently witnessing the very first of many tailor-made vaccines being rolled out for general use, so don’t expect the COVID-19 jabs to be the only vaccines coming our way. With a 20 to 1 return on investment on many of these new technologies, most pharmaceutical giants will surely be lobbying governments across the globe for the next “necessary” vaccination program. The idea of multiple COVID-19 vaccinations throughout the year is already being presented as a very possible outcome for the future of humanity. Yet, no studies have been completed showing the risk of taking different types of vaccines. There have also been suggestions that people will have to have the same vaccine that they had previously taken every six months or so. This will leave Astrazeneca, Pfizer and Moderna picking up repeat vaccine contracts worth billions in secured future revenue before there are any real data on the results of the vaccines.
7. No interaction studies have been performed and there are no, or a limited amount of, data from the use of COVID-19 mRNA Vaccine BNT162b2
Admissions like these should be a cause for concern for anybody reading the official guidance. While officials and carefully chosen “trusted sources” are telling you that “no corners have been cut” in the race to approve these vaccines, it is also true that no full length studies have been completed either. These two facts are juxtaposed and obviously contradict the official narrative that is being thrust upon the general public by all of those involved.
It is clear that the officials have no real data on what will happen next and that there is a tsunami of ethical questions that are not being answered. In the absence of data, there will be speculation.
8. It is unknown whether COVID-19 mRNA Vaccine BNT162b2 is excreted in human milk and It is unknown whether COVID-19 mRNA Vaccine BNT162b2 has an impact on fertility
It is vital to note the potential dangers posed by the BNT162b2 to unborn and newborn babies as well as the reproductive organs in general. There are so many parts of the Pfizer/BioNTech clinical trials that have not yet been completed. Dr. Peter Klatsky, the Director of Fertility Preservation at the Bay Area’s Spring Fertility, talking about the coming animal trials which are to be performed over the coming months was quoted in SFGate as saying, “It will reassure me an awful lot if the protein expression is not seen on the placenta. That the mRNA isn’t making it to the placenta in animals,” he said. “I don’t expect to see any.” The article goes on to explain that it will be about another 9 months until the data has been collected and analyzed.

Big names in mainstream media have also been caught recklessly promoting the vaccine to pregnant women, such as Karen Weintraub writing for USA Today, whose recent article quickly states, “Although there are very little data on how pregnant and nursing mothers will respond to a COVID-19 vaccine, professional organizations and individual doctors say the benefits are very likely to outweigh the risks.” Even though the clinical trials intentionally excluded pregnant women, Weintraub went on to state that “23 women in the Pfizer-BioNTech trial and 13 in Moderna’s became pregnant during the trial.”
While the UK’s official guidance is left sounding ambiguous, on the European continent, the European Medicines Agency (EMA) states that “the Pfizer vaccine should be considered on a case by case basis for pregnant women”, but they also reserve the right to alter the guidance if more data becomes available. It seems there is no longer any erring on the side of caution with some regulators when it comes to the COVID-19 vaccinations.
9. Non-clinical data reveal no special hazard for humans based on a conventional study of repeat dose toxicity but animal studies into potential toxicity to reproduction and development have not been completed
Animal studies have not been completed and, as referred to in the previous section, the data on those animal trials will not be available for another 9 months. It is, of course, a very rare decision to approve an experimental medical technology before any animal studies have been completed. This should be a great cause for concern for any free thinking man or woman. The fact that they have had to use what they refer to as “non-clinical” data in these studies is also in conflict with the idea that the trials were conducted to the highest professional standard. The document also fails to clearly define what non-clinical data actually means.
10. In the absence of compatibility studies, this medicinal product must not be mixed with other medicinal products
Possibly the most fascinating admission in the entire document is the absence of any compatibility studies when somebody is given the vaccine while on any other medication or medical treatment. The guidance clearly states “this medicinal product should not be mixed with other medical products.” This completely jaw dropping sentence will lead many to assume that if you are on any medication at all, then you shouldn’t be given the vaccine. Whether this refers to the mixing of other medical properties directly together with the vaccine, or simultaneous dosing of any other medical product is unclear from the official guidance.
The Mail Online and The Guardian reported in 2019 that a staggering 1 in 4 people in England – nearly 12 million people – were taking what was described as “addictive” prescription medicines such as antidepressants, sleeping pills and opioid painkillers, saying that “the NHS must take action”. Those statistics throw into question the mass rollout of a vaccination with no compatability studies. This makes the fact that elderly care home residents, followed by those aged over 80, will be the first to recieve the experimental Pfizer vaccine an extremely risky strategy. Also in 2019, Age UK reported that nearly 2 million older people were on more that 7 prescription medicines and were at “risk of side effects that are severe in some cases, and occasionally even life threatening.” This worrying issue has been barely reported by the “trusted news sources”.
A Conclusive Lack of Real Data
After examining the official guidance, one fact becomes glaringly obvious — there is little to no data on the official Pfizer vaccine in key areas. In the clinical trials, children as young as 12 years old were used as unnecessary guinea pigs. There also wasn’t enough care taken to avoid pregnant women being involved in the initial clinical trials and under the cover of unyielding and uneducated mainstream propaganda, the safety of some of the most vulnerable people involved in the vaccine trials have been ignored by Pfizer and the politicians who have successfully pushed for the public vaccination campaign to essentially replace mass clinical trials. The stage has been set for a potential disaster on an unimaginable scale. It isn’t only the participants of the trials who are risking their health for the sake of big pharmaceutical companies’ hyperinflated profit margin, but it is also the medical professionals who could be risking their futures by collaborating in these risky experimental trials, which will certainly see many people dead and irreversibly injured.
In one section of Reg 174, the Big Pharma giant lays out the risk to people’s health from the Pfizer/BioNTech vaccine. The most common adverse reaction in participants 16 years of age and older was pain at the injection site, which affected a massive 80% of those taking part in the Pfizer trials. Fatigue came a close second with 60% of trial participants becoming sluggish and tired. Half of those involved in the studies suffered from a headache as the experimental vaccine went to work while myalgia was experienced by 30% of vaccine recipients, though the results do not indicate whether the myalgia was acute (short-term) or chronic (long-term). Almost a third of participants came down with chills, while just under 1 in 5 people suffered from arthralgia (joint pain) and 1 in 10 from pyrexia (increased body temperature).
Adverse reactions reported in clinical trials are listed in the study in decreasing order of frequency and seriousness. Just under 1 in 10 people who take the vaccine will suffer from the very common and common adverse reactions referred to in the latter paragraph, such as headaches, myalgia and chills, but the more serious issues are classified as uncommon – including Lymphadenopathy (which causes swollen or enlarged lymph nodes) and nervous system disorders – which may affect up to 1 in 100 people. Rare adverse reactions that could affect up to 1 in 1000 people and very rare adverse reactions that would affect less than 1 in 10,000 of the vaccine recipients were not included in Pfizer’s self-reported safety information. It has obviously been decided that this information should be kept out of the public domain as much as possible to avoid any further vaccine hesitancy.
Not only does the official guidance actively hide the types of rare and very rare adverse effects, but they have also been leaving out some of the adverse reactions reported during the clinical trials. As I write this, the Reg 174 guidance for healthcare professionals is on version 10.1 of the document and, since its release, they have yet to admit to the potential of a certain uncommon adverse reaction to the vaccine being a specific nervous system disorder. Structural nervous system disorders include brain or spinal cord injury, Bell’s palsy, cervical spondylosis, carpal tunnel syndrome, brain or spinal cord tumors, peripheral neuropathy, and Guillain-Barré syndrome. However, previous versions of the guidance gives no clue as to what type of nervous system disorders they were referring to. However, recent articles in the USA Today, heavily promoted by the Microsoft Network, suggested that the Bell’s palsy some people came down with in the vaccine trials wasn’t related to the Pfizer jab. The article states that on Dec. 10, the FDA’s Center for Biologics Evaluation and Research held the 162nd meeting of the Vaccines and Related Biological Products Advisory Committee to discuss the emergency use authorization of the Pfizer-BioNTech COVID-19 vaccine. The USA Today piece even goes on to admit that , “a 53-page briefing noted that there had been four cases of Bell’s palsy among the vaccinated group and none among the placebo group.”
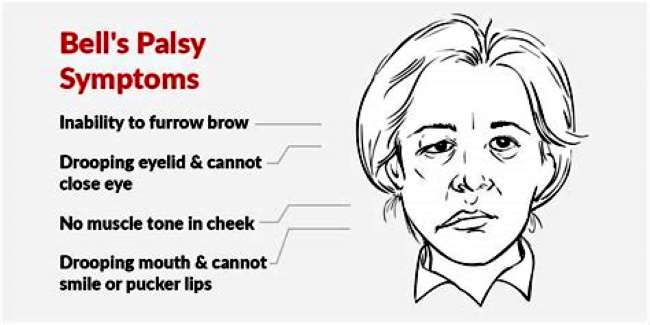
Even though Miriam Fauzia, who wrote the USA Today piece, claims that the Bell’s palsy was not related to the experimental Pfizer vaccine, the 53-page briefing she sources clearly states, “Among non-serious unsolicited adverse events, there was a numerical imbalance of four cases of Bell’s palsy in the vaccine group compared with no cases in the placebo group, though the four cases in the vaccine group do not represent a frequency above that expected in the general population.” While it is true that 1 to 4 people in 10,000 will develop Bell’s palsy within the general population, it should be noted that the 4 cases in the vaccine trials and none in the placebo group makes for a statistical anomoly that must be examined more thoroughly. Instead, the mainstream media moved quickly to discredit the Bell’s palsy links to the Pfizer vaccine using various mislead tactics to achieve their aims.
Many mainstream outlets were caught spouting the same misleading information with articles entitled “Why you shouldn’t worry about a connection between Bell’s palsy and COVID-19 vaccines,” from Business Insider and a Reuters article from 14 December 2020 entitled, “Fact check: Photo does not show three recipients of Pfizer’s COVID-19 vaccine that developed Bell’s palsy.”
In the case of the Reuters article, which is described as written by “Reuters Staff” rather than a specific journalist, the focus was not on the four Pfizer clinical trial participants who developed Bell’s palsy but instead the article discredits a random post on social media of three people with Bell’s palsy unconnected to the Pfizer vaccine. These type of misinforming mainstream media articles are commonly found to be using obvious fallacies to mislead their readership and with no individual taking responsibility for writing the misinforming piece, a trick repeated by many other media companies complicit with the official narrative. The Reuters article even goes on to admit that: “According to the FDA’s briefing document dated December 10, Bell’s palsy was reported in four vaccine participants and none in the placebo group, out of the 44,000 total participants of the late-stage vaccine trial.” However, the title of the Reuters article would mislead even some of the most keen eyed observers.
The mainstream media has been creating a flood of misleading stories, but it appears as though they have been given carte blanche to continue to do so, probably because they are sticking so tightly to the official narrative. It’s a narrative that is thick with irony, for it is the “trusted sources” who are being caught systematically misleading the general population again and again while also declaring a propaganda war against “fake news”.
The official guidance noted in Reg 174 doesn’t only highlight the serious lack of real data gained from Pfizer’s clinical trials for its Covid-19 vaccine so far, but it also exposes the wealthy medical professionals involved in these experimental vaccine development programs as complacent, reckless and very naive. It’s no secret that children are, more often than not, incapable of giving informed legal consent for such a risky and unethical enterprise. But the pro-vax extremists are using every tactic to coerce and manipulate children and their guardians into becoming human guinea pigs for Big Pharma. Pregnant women are also treated as acceptable collateral damage to advance the new science of gene, mRNA and DNA manipulation, a science and technology that pushes a sinister transhumanist agenda.
Don’t be fooled by the carefully worded vacuous celebrities, self-serving politicians, Big Pharma, and the mainstream medias authoritarian style misinformation campaigns. Keep your humanity intact and read their own words. The government guidance to healthcare professionals clearly states on multiple occasions that there are “no data available”.

Truth Comes to Light highlights writers and video creators who ask the difficult questions while sharing their unique insights and visions.
Everything posted on this site is done in the spirit of conversation. Please do your own research and trust yourself when reading and giving consideration to anything that appears here or anywhere else.










