Nearly 30,000 Deaths After COVID Vaccines Reported to VAERS, CDC Data Show
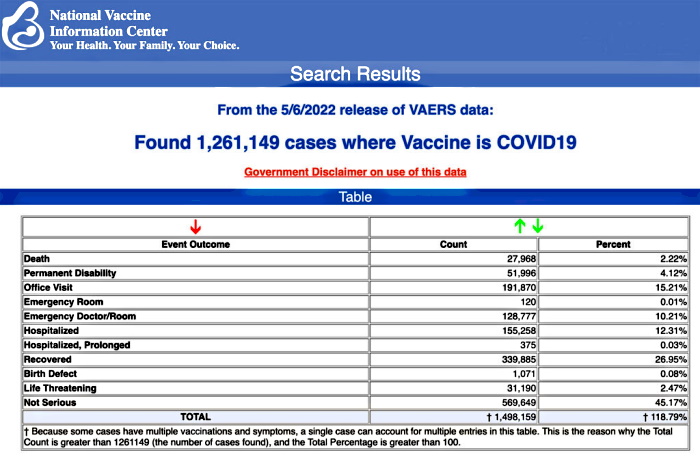
VAERS data released Friday by the Centers for Disease Control and Prevention show 1,261,149 reports of adverse events from all age groups following COVID-19 vaccines, including 27,968 deaths and 228,477 serious injuries between Dec. 14, 2020, and May 6, 2022.
by Megan Redshaw, The Defender
May 13, 2022
The Centers for Disease Control and Prevention (CDC) today released new data showing a total of 1,261,149 reports of adverse events following COVID-19 vaccines were submitted between Dec. 14, 2020, and May 6, 2022, to the Vaccine Adverse Event Reporting System (VAERS). VAERS is the primary government-funded system for reporting adverse vaccine reactions in the U.S.
The data included a total of 27,968 reports of deaths — an increase of 210 over the previous week — and 228,477 serious injuries, including deaths, during the same time period — up 1,774 compared with the previous week. There were 5,794 additional total adverse events reported to VAERS over the previous week.
Excluding “foreign reports” to VAERS, 815,384 adverse events, including 12,899 deaths and 81,830 serious injuries, were reported in the U.S. between Dec. 14, 2020, and May 6, 2022.
Foreign reports are reports foreign subsidiaries send to U.S. vaccine manufacturers. Under U.S. Food and Drug Administration (FDA) regulations, if a manufacturer is notified of a foreign case report that describes an event that is both serious and does not appear on the product’s labeling, the manufacturer is required to submit the report to VAERS.
Of the 12,899 U.S. deaths reported as of May 6, 16% occurred within 24 hours of vaccination, 20% occurred within 48 hours of vaccination and 59% occurred in people who experienced an onset of symptoms within 48 hours of being vaccinated.
In the U.S., 578 million COVID-19 vaccine doses had been administered as of May 6, including 341 million doses of Pfizer, 218 million doses of Moderna and 19 million doses of Johnson & Johnson (J&J).
Every Friday, VAERS publishes vaccine injury reports received as of a specified date. Reports submitted to VAERS require further investigation before a causal relationship can be confirmed.
Historically, VAERS has been shown to report only 1% of actual vaccine adverse events.
U.S. VAERS data from Dec. 14, 2020, to May 6, 2022, for 5- to 11-year-olds show:
- 10,560 adverse events, including 272 rated as serious and 5 reported deaths.
- 20 reports of myocarditis and pericarditis (heart inflammation).
The CDC uses a narrowed case definition of “myocarditis,” which excludes cases of cardiac arrest, ischemic strokes and deaths due to heart problems that occur before one has the chance to go to the emergency department.
The Defender has noticed over previous weeks that reports of myocarditis and pericarditis have been removed by the CDC from the VAERS system in this age group. No explanation was provided. - 43 reports of blood clotting disorders.
U.S. VAERS data from Dec. 14, 2020, to May 6, 2022, for 12- to 17-year-olds show:
- 31,504 adverse events, including 1,812 rated as serious and 43 reported deaths. VAERS reported 44 deaths in the 12- to 17-year-old age group last week.
- 65 reports of anaphylaxis among 12- to 17-year-olds where the reaction was life-threatening, required treatment or resulted in death — with 96% of cases attributed to Pfizer’s vaccine.
- 650 reports of myocarditis and pericarditis with 638 cases attributed to Pfizer’s vaccine.
- 166 reports of blood clotting disorders with all cases attributed to Pfizer.
U.S. VAERS data from Dec. 14, 2020, to May 6, 2022, for all age groups combined, show:
- 20% of deaths were related to cardiac disorders.
- 54% of those who died were male, 41% were female and the remaining death reports did not include the gender of the deceased.
- The average age of death was 73.
- As of May 6, 5,503 pregnant women reported adverse events related to COVID-19 vaccines, including 1,720 reports of miscarriage or premature birth.
- Of the 3,629 cases of Bell’s Palsy reported, 51% were attributed to Pfizer vaccinations, 40% to Moderna and 8% to J&J.
- 873 reports of Guillain-Barré syndrome, with 42% of cases attributed to Pfizer, 30% to Moderna and 29% to J&J.
- 2,331 reports of anaphylaxis where the reaction was life-threatening, required treatment or resulted in death.
- 1,698 reports of myocardial infarction.
- 13,922 reports of blood-clotting disorders in the U.S. Of those, 6,248 reports were attributed to Pfizer, 4,972 reports to Moderna and 2,661 reports to J&J.
- 4,183 cases of myocarditis and pericarditis with 2,562 cases attributed to Pfizer’s, 1,424 cases to Moderna’s and 184 cases to J&J’s COVID-19 vaccines.
Pfizer’s COVID efficacy fades rapidly just weeks after second and third doses
Second and third doses of Pfizer’s COVID-19 vaccine provide protection against the Omicron variant for only a few weeks, according to peer-reviewed research published today in JAMA Network Open.
“Our study found a rapid decline in Omicron-specific serum neutralizing antibody titers only a few weeks after the second and third doses of [the Pfizer-BioNTech] BNT162b2,” the authors of the research letter wrote.
The authors said their findings “could support rolling out additional booster shots to vulnerable people as the variant drives an uptick in new cases across the country,” Forbes reported.
Danish researchers studied adults who received two or three doses of BNT162b2 between January 2021 and October 2021, or were previously infected prior to February 2021 and then vaccinated.
They found that after an initial increase in Omicron-specific antibodies after the second Pfizer shot, levels dropped rapidly, from 76.2% at week 4, to 53.3% at weeks 8 to 10, and 18.9% at weeks 12 to 14.
After the third shot, neutralizing antibodies against Omicron fell 5.4-fold between week 3 and week 8.
COVID vaccines for kids under 6 won’t have to meet FDA 50% efficacy standard
The FDA’s top vaccine official told a congressional committee on May 6 that COVID-19 vaccines for kids under 6 will not have to meet the agency’s 50% efficacy threshold for blocking symptomatic infections required to obtain Emergency Use Authorization.
“If these vaccines seem to be mirroring efficacy in adults and just seem to be less effective against Omicron like they are for adults, we will probably still authorize,” Dr. Peter Marks, director of the Center for Biologics Evaluation and Research at the FDA told the House Select Subcommittee on the Coronavirus Crisis.
The FDA is reviewing data from Moderna’s two-shot vaccine for infants and toddlers 6 months to 2 years old, and for children 2 to 6 years old. The company asked the FDA on April 28 to approve its COVID-19 mRNA-1273 vaccine for children, citing different efficacy numbers than it disclosed in March.
The FDA is still awaiting data on Pfizer and BioNTech’s three-dose regimen for children under age 5 after two doses of its pediatric vaccine failed to trigger an immune response in 2-, 3- and 4-year-olds comparable to the response generated in teens and adults.
COVID vaccine injury ends surgeon’s 20-year career
In an interview on CHD.TV’s “The People’s Testaments,” Dr. Joel Wallskog described how he was diagnosed with transverse myelitis after getting the Moderna COVID-19 vaccine, and why he now devotes his time to helping others injured by the vaccine.
In September 2020, Wallskog said, staff members in the clinic he referred patients to began coming down with COVID-19. Although Wallskog did not feel ill, he got an antibody test and it was positive.
When a close friend came down with COVID-19 and had to be intubated, Wallskog decided he should get vaccinated, despite reservations and having already acquired natural immunity.
About a week after receiving his vaccine, Wallskog’s feet became numb and he developed “electrical sensations” down his legs when he bent his head forward. When he began having trouble standing, he ordered emergent MRIs and was found to have a lesion on his spinal cord.
A neurologist diagnosed Wallskog with transverse myelitis, a disorder caused by inflammation of the spinal cord.
Despite various treatments and rest, Wallskog suffers pain and numbness and is unable to stand long enough to perform surgery. His career came to an end in early 2021.
Rheumatologist: 40% of 3,000 vaccinated patients reported vaccine injury
Dr. Robert Jackson, a practicing rheumatologist for 35 years said 40% of the vaccinated patients in his practice reported a vaccine injury, and 5% are still injured. Jackson has more than 5,000 patients, about 3,000 of whom received a COVID-19 vaccine.
Jackson said he’s had 12 patients die following the shot, whereas he normally sees one or two deaths in his patient base a year. About 5% of his patients developed a new condition that makes them susceptible to blood clotting.
Jackson’s observations are consistent with a study published in the BMJ that assessed the safety of vaccines against SARS-CoV-2 in people with inflammatory/autoimmune rheumatic and musculoskeletal disease from the EULAR Coronavirus Vaccine (COVAX) physician-reported registry.
The study showed 37% of 5,121 participants had adverse events and 4.4% of patients had a flare-up of their disease after vaccination.
Children’s Health Defense asks anyone who has experienced an adverse reaction, to any vaccine, to file a report following these three steps.
©May 2022 Children’s Health Defense, Inc. This work is reproduced and distributed with the permission of Children’s Health Defense, Inc. Want to learn more from Children’s Health Defense? Sign up for free news and updates from Robert F. Kennedy, Jr. and the Children’s Health Defense. Your donation will help to support us in our efforts.

Truth Comes to Light highlights writers and video creators who ask the difficult questions while sharing their unique insights and visions.
Everything posted on this site is done in the spirit of conversation. Please do your own research and trust yourself when reading and giving consideration to anything that appears here or anywhere else.










