CHD to Sue FDA for ‘Recklessly Endangering’ Children if Agency Authorizes Pfizer Vaccine for Children 5 to 11 Years Old
An advisory committee to the U.S. Food and Drug Administration will meet Tuesday to consider emergency authorization of Pfizer’s COVID vaccine for young children. In a letter to the FDA, Children’s Health Defense outlines why such a move would be reckless.
by Children’s Health Defense Team
October 25, 2021
Children’s Health Defense (CHD) today said it will take legal action against the U.S. Food and Drug Administration (FDA) if the agency grants Emergency Use Authorization (EUA) for the Pfizer-BioNTech SARS-CoV-2 vaccine for children aged 5-11.
In a letter signed by Robert F. Kennedy, Jr., CHD chairman and chief legal counsel, and Dr. Meryl Nass, CHD board member, Kennedy and Nass wrote:
“CHD will seek to hold you accountable for recklessly endangering this population with a product that has little efficacy but which may put them, without warning, at risk of many adverse health consequences, including heart damage, stroke, and other thrombotic events and reproductive harms.”
The letter was addressed to Dr. Arnold Monto, chairman of the FDA’s Vaccines and Related Biological Products Advisory Committee (VRBPAC), committee members and all FDA staff.
VRBPAC members are set to meet Tuesday to consider and likely vote on whether to grant EUA for the Pfizer vaccine for 5- to 11-year olds.
In May, the FDA authorized Pfizer’s vaccine for 12- to 15-year-olds. Moderna and Johnson & Johnson vaccines have not yet been authorized for children under 18.
The letter outlines 12 reasons the FDA should not authorize the pediatric vaccine and provides supporting evidence to back up each argument.
Read the letter:
Dear Chairman Monto, VRBPAC Members and FDA Staff:
We write to you on behalf of Children’s Health Defense (CHD), a non-profit organization devoted to the health of people and the planet. We have actively followed your work to evaluate, authorize and approve vaccines for the American public and particularly children.
We are aware that you are likely to authorize Pfizer’s BioNTech SARS-CoV-2 vaccine for children aged 5-11 at your meeting on Oct. 26. Your authorization thus will expose over 20 million children in the U.S., and millions more around the world, to potential COVID-19 vaccination of an Emergency Use Authorization (EUA) product.
We are writing to put you on notice that should you grant EUA status to this pediatric EUA vaccine, CHD is poised to take legal action against you and other Vaccines and Related Biological Products Advisory Committee (VRBPAC) voting members as well as the FDA.
CHD will seek to hold you accountable for recklessly endangering this population with a product that has little efficacy but which may put them, without warning, at risk of many adverse health consequences, including heart damage, stroke and other thrombotic events and reproductive harms.
We briefly outline why such authorization would be reckless:
1. The risks demonstrably outweigh the benefits of COVID vaccination for young children. Deaths and hospitalizations are rare and have been inflated inaccurately.
2. Nearly half of all children have natural immunity to COVID, according to the Centers for Disease Control and Prevention (CDC). There is no ethical justification for superfluous vaccination that will put children at elevated risk of vaccine harm.
3. Some children likely will die or be permanently injured from these vaccines based on the authorization for children 12-16.
4. The clinical trials for the pediatric vaccine were too small to detect safety signals for a population in the millions.
5. There are no long-term safety data for COVID vaccination of young children, making this an experiment rather than appropriate medical prevention.
6. Unethical coercive pressure will be applied to children and their parents, as has occurred with older children and adults. To grant authorization is to abet this unethical coercion that violates the Nuremberg Code’s first principle.
7. There is no available care for children injured by COVID shots. The science and medicine have not yet developed, and most families will be unable to cover the costs of potential catastrophic injuries.
8. VRBPAC members should not participate in an exercise disguising a foregone conclusion. The president’s purchase of 65 million pediatric doses, the CDC guidance for COVID vaccine delivery, the American Academy of Pediatrics’s promotion of COVID vaccination for children all call into question whether this committee’s deliberations mean anything.
If the administration is unprepared to wait for your advice, let alone heed it, you should signify your disapproval on behalf of the country the FDA is meant to protect.
9. First, do no harm. You are physicians who owe a duty to patients and medical ethics. If you authorize these shots, given all you know, will you be upholding your oath? If not, is it possible that your acts could later be seen as reason to remove your medical licenses?
10. The liability-free nature of your deliberations may not stand the test of time. In the fullness of time, your decisions may not have the liability protection that they currently enjoy. Under the PREP Act of 2005, all actors advancing an EUA agenda for medical countermeasures enjoy liability protection, absent willful misconduct.
Nonetheless, if at a later point these shots are deemed non-therapeutic gene products that you knowingly and recklessly authorized, and which were then distributed to children as a direct result of your decision, it is possible that liability could later attach.
11. There is no COVID emergency for children of this age.
12. There are safer drugs that could be used prophylactically and therapeutically for COVID in children. There is extensive and compelling medical evidence for this assertion — and the choice to eschew use of these drugs in favor of a demonstrably dangerous vaccine is arbitrary and capricious.
We ask that you carefully consider all the information above before making any recommendation to authorize Pfizer’s vaccine in the 5 through 11 year age group at your meeting on Tuesday, Oct. 26.
Sincerely yours,
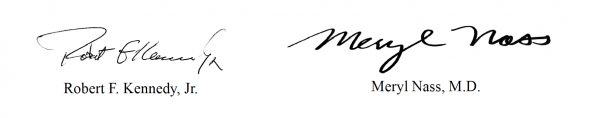
Read the full article at Children’s Health Defense
©October 2021 Children’s Health Defense, Inc. This work is reproduced and distributed with the permission of Children’s Health Defense, Inc. Want to learn more from Children’s Health Defense? Sign up for free news and updates from Robert F. Kennedy, Jr. and the Children’s Health Defense. Your donation will help to support us in our efforts.
Connect with Children’s Health Defense
cover image credit: KlausHausmann / pixabay

Truth Comes to Light highlights writers and video creators who ask the difficult questions while sharing their unique insights and visions.
Everything posted on this site is done in the spirit of conversation. Please do your own research and trust yourself when reading and giving consideration to anything that appears here or anywhere else.










