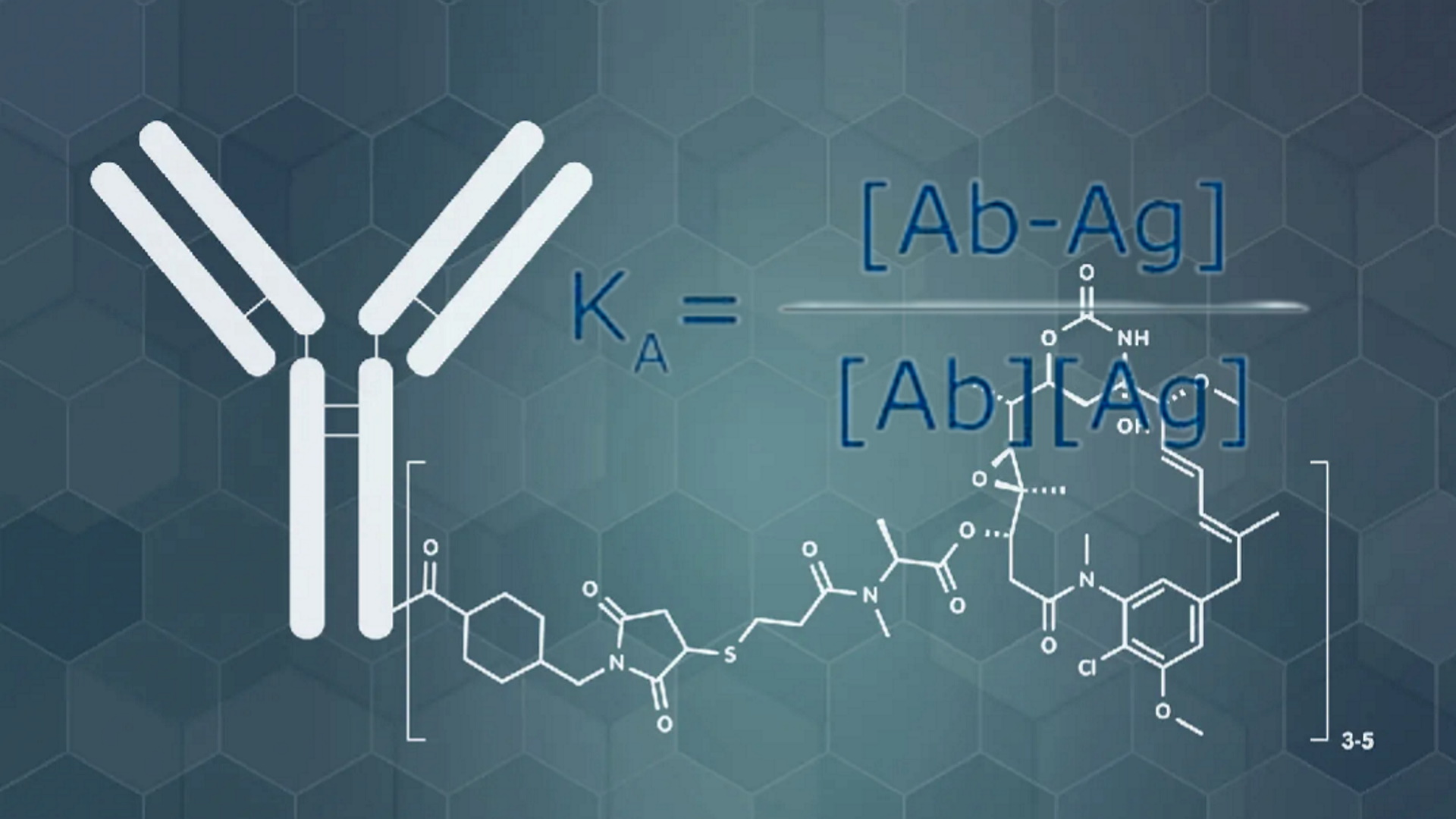
The Antibody Equation (1929): “Antibodies Were (and Still Are) Nothing More Than Unseen Theoretical Constructs”
by Mike Stone, ViroLIEgy
May 7, 2022
It is very apparent to anyone looking into the origins of antibodies that the idea of what these entities are in terms of how they look and how they function came well before any attempts to actually purify, isolate, and characterize the assumed particles. Antibodies were (and still are) nothing more than unseen theoretical constructs used to explain chemical reactions created in a lab. These fictional creations reside in the “domain of the invisible spectrum” conjured up by the “lively imagination” of a man named Paul Ehrlich. While there was no direct proof for the existence of these entities, the antibody concept was far too important to the immunological narratives forming around the growing practice of vaccination and the increased acceptance of other unseen entities known as “viruses” to just give it up. As the purification and isolation of antibodies in order to see and study them was an impossible task, researchers sought other methods to attempt to provide indirect evidence for the existence of these theoretical creations.
One man who is credited with providing such evidece is Michael Heidelberger, considered the “Founder of Immunochemistry.” He was the first to apply mathematics to the reaction of antibodies and their antigens. He is also known for “proving” that antibodies are proteins by showing that the antigens of pneumococcus bacteria are polysaccharides (or carbohydrates). Here is a brief overview of his work:
How Heidelberger and Avery sweetened immunology
All about nitrogen
“Avery and Dochez’s initial characterization of this pneumococcal substance showed that it was resistant to both heat and trypsin—features unbefitting most proteins—but that it did contain nitrogen, a component of proteins. But its true nature was not revealed until 1923, when Michael Heidelberger—then in the chemistry department synthesizing drugs against poliomyelitis and African sleeping sickness—teamed up with Avery.
The more they purified the reactive substance the less nitrogen it contained. When it was virtually nitrogen-free, recalled Heidelberger in a 1979 article, Avery ventured a guess: “Could it be a carbohydrate?” (2). Chemical analysis confirmed its sugary character, and subsequent studies of other pneumococcal serotypes revealed that each bacterial capsule had a distinct polysaccharide signature. It was this signature that dictated the serological specificity of the organism. The duo published these findings in two articles in the Journal of Experimental Medicine (3, 4).
Their results were met with considerable skepticism, as it was then thought that only proteins could incite a specific immune response. “Nobody believed it,” says Emil Gotschlich (Rockefeller University), whose later work on polysaccharide-based vaccines stemmed in large part from Heidelberger and Avery’s discoveries. “It took them a lot of effort to convince people that the polysaccharide was the immunoreactive component.”
Antibodies solidified
Heidelberger and Avery’s discovery came at a time when antibodies were regarded—by those who believed they existed at all—as mysterious substances that floated around in serum. “It appeared to me that there was a crying need to determine the true nature of antibodies,” wrote Heidelberger in 1979, “and that until this was done there could be no end to the polemics and uncertainties that were plaguing immunology” (2). Heidelberger later purified the antibodies from his precipitin reactions and showed that they themselves were proteins. As a result, says friend and colleague Victor Nussenzweig (New York University), “there were no more mystical ideas about what antibodies were.”
Heidelberger and his postdoctoral fellow Forrest Kendall later quantitated the precipitin reaction (5), bringing much-needed mathematics to the study of antibody–antigen interactions and lifting antibodies even further out of the realm of the mysterious (see the next “From the Archive”).”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2212983/#!po=46.8750

Two of Heidelberger’s papers are most often cited as the proof that antibodies are proteins. The first is a paper he did with Oswald Avery in 1923. It is used as proof that the pneumococcus antigens are carbohydrates. However, was this paper successful in drawing this conclusion? Presented here are some highlights from their collaboration:
The Soluble Specific Substance of Pneumococcus.
“In 1917 Dochez and Avery (1) showed that whenever pneumococci are grown in fluid media, there is present in the cultural fluid a substance which precipitates specifically in antipneumococcus serum of the homologous type. This soluble substance is demonstrable in culture filtrates during the initial growth phase of the organisms; that is, during the period of their maximum rate of multiplication when little or no cell death or disintegration is occurring. The formation of this soluble specific material by pneumococci on growth in vitro suggested the probability of an analogous substance being formed on growth of the organism in the animal body.
Examination of the blood and urine of experimentally infected animals gave proof of the presence of this substance in considerable quantities in the body fluids following intraperitoneal infection with pneumococcus. In other words, this soluble material elaborated at the focus of the disease readily diffuses throughout the body, is taken up in the blood, passes the kidney, and appears in the urine unchanged in specificity. Similarly, a study of the serum of patients suffering from lobar pneumonia has revealed a substance of like nature in the circulating blood during the course of the disease in man. Furthermore, examination of the urine of patients having pneumonia due to pneumococci of Types I, II, and III has shown the presence of this substance in some stage of the disease in approximately two-thirds of the cases.
Recently from filtered alkaline extracts of pulverized bacteria of several varieties, including pneumococci, Zinsser and Parker have prepared substances which appear free from coagulable protein. These substances, called “residue antigens,” are specifically predpitable by homologous antisera. These observers consider these acid- and heat-resistant antigenic materials analogous to the soluble specific substance of pneumococcus described by Dochez and Avery. In spite of the fact that these “residue antigens” are precipitable by homologous sera produced by immunization with the whole bacteria, Zinsser and Parker have so far failed to produce antibodies in animals by injecting the residues.
In the earlier studies by Dochez and Avery certain facts were ascertained concerning the chemical characteristics of this substance. It was found that the specific substance is not destroyed by boiling; that it is readily soluble in water, and precipitable by acetone, alcohol, and ether; that it is precipitated by colloidal iron, and does not dialyze through parchment; and that the serological reactions of the substance are not affected by proteolytic digestion by trypsin. Since the substance is easily soluble, thermostable, and type-specific in the highest degree, it seemed an ideal basis for the beginning of a study of the relation between bacterial specificity and chemical constitution. The present report deals with the work done in this direction.
Experimental
The organism used in the present work was Pneumococcus Type II. The most abundant source of the soluble specific substance appeared to be an 8 day autolyzed broth culture; hence this material was used as the principal source of supply. For comparison dissolved pneumococci and lots of urine containing the specific substance were also worked up, with essentially the same results, as will be seen from Table I.
The process for the isolation of the soluble specific substance consisted in concentration of the broth, precipitation with alcohol, repeated re-solution and reprecipitation, followed by a careful series of fractional precipitations with alcohol or acetone after acidification of the solution with acetic acid, and, finally, repeated fractional precipitation with ammonium sulfate and dialysis of the aqueous solution of the active fractions.
Five lots of 15 liters each of 8 day cultures of Pneumococcus Type II in meat infusion phosphate broth are each concentrated on the water bath in large evaporating dishes to 1,000 to 1,200 cc. and precipitated in a separatory funnel by the gradual addition, with vigorous rotation, of 1.2 volumes of 95 per cent alcohol.The mixture separates into two layers, and is allowed to stand over night, or for several hours.
The upper layer, which is almost black and comprises the largest part of the mixture, contains only traces of the soluble specific substance, and is siphoned off and discarded. The lower, more viscous layer is run into a 250 cc. centrifuge bottle (occasionally a second will be required), capped, and rotated at high speed for ½ hour. Three layers are formed, of which the uppermost is merely a further amount of the liquid previously discarded. The middle layer consists of a compact, greenish cake of insoluble matter and gummy material, and contains most of the soluble specific substance. The bottom layer, from which salts often separate, is a brownish syrup rich in salts and nitrogenous matter and relatively poor in specific substance, and can, by careful manipulation, be poured off to a large extent.
Although a small proportion of the specific substance is lost if this syrup is discarded, its elimination represents so considerable a purification as to warrant the sacrifice of the active material contained. The gummy cake remaining in the centrifuge bottle, together with adhering salts and syrup, is now rinsed out and ultimately combined with similar material from the other lots, All of this is then dissolved as completely as possible in water, care being taken to break up the many lumps of gummy material, diluted to 1 liter, and again precipitated with alcohol. In this case about 1.3 liters are required to precipitate all but the last traces of active material from the upper layer. This is again discarded and the lower layer treated as before. At this stage there is relatively less of the bottom layer, and it is more difficult to separate it from the cake containing the specific substance, but as much as possible is removed. The remaining material is smoothed out with water, diluted to about 500 cc., and centrifuged. The precipitate is washed twice with water, and the washings are combined with the main solution. The still turbid liquid, the volume of which should be about 750 cc., is put through the alcohol purification process a third time, about 1.1 liters of alcohol being required. After having been centrifuged, the active material is again dissolved in water, made definitely acid to litmus with acetic acid, and again centrifuged. The precipitate is washed three times with water acidulated with acetic acid, and the filtrate and washings are combined in a separatory funnel and diluted again if necessary to 750 cc. Acetone (redistilled) is now added until a permanent precipitate forms, about 250 c¢. being necessary. The precipitate is allowed to settle, whereupon the lower part of the mixture containing the precipitate is drawn off and centrifuged. The clear superuatant fluid is restored to the main solution, while the precipitate, which consists largely of insoluble material and gives an aqueous solution almost devoid of activity, is discarded.
Fractional precipitation is continued, and even when the specific substance appears in quantity in the precipitate, it is occasionally possible to separate a lower, inactive, syrupy layer, as in the previous purifications by alcohol. Addition of acetone is continued until a test portion, heated on the water bath to remove acetone, diluted with saline, and neutralized, no longer gives a precipitate with immune serum, after which the upper layer may be discarded. The active precipitates are then redissolved in water, centrifuged again, and the supernatant liquid is diluted to 375 cc., reacidified with acetic acid, and again fractionated with acetone. If inactive fractions are obtained, the process is again repeated until no further purification results. Alcohol may be used for these fracfionations instead of acetone, the only difference being that a somewhat larger proportion is required. The active material is then dissolved in about 150 cc. of water and again made definitely acid with acetic acid. The solution is treated with solid ammonium sulfate until the first slight precipitate forms. This is generally inactive, and if so, may be discarded.
Finally, ammonium sulfate is added to saturation, completely precipitating the specific substance if the volume of the solution is not too great. The mixture is allowed to stand for several hours and is then centrifuged and the precipitate washed with a little saturated ammonium sulfate solution. It is redissolved in about 75 cc. of water acidified with acetic acid, centrifuged if necessary, and again precipitated by saturation with ammonium sulfate. Finally, the specific substance so obtained is dissolved in water and dialyzed first against running tap water in the presence of chloroform and toluene, and finally against distilled water until tests for sulfate and phosphate ion are negative. Addition of acetic acid during the early stages of the dialysis assists in the removal of calcium, which otherwise forms a large part of the ash.
The dialyzed solution is concentrated to dryness on the water bath and the residue redissolved in hot water. If the solution is not perfectly clear, it is centrifuged again before being evaporated to dryness, and the whole process is repeated as long as insoluble material separates. Toward the end of the final concentration absolute alcohol may be added to assist in the precipitation of the substance.
Variations in the exact volumes given are often necessary with different lots of broth, but this will occasion little difficulty if all fractionations are controlled by the specific precipitin test.
As so obtained the soluble specific substance forms an almost colorless varnish-like mass which may be broken up and dried to constant weight at 100°C. in vacuo. The yield from 75 liters of broth averages about 1 gin., although it varies within rather wide limits in individual lots.
By the method outlined above all substances precipitable with hosphotungstic acid or capable of giving the biuret reaction were eliminated. The residual material (Preparation 17, in Table I), for which no claim of purity is made, as efforts at its further purification are still under way, contained, on the ash-free basis, 1.2 percent of nitrogen. It was essentially a polysaccharide, as shown by the formation of 79 percent of reducing sugars on hydrolysis, and by the isolation and identification of glucosazone from the products of hydrolysis.”
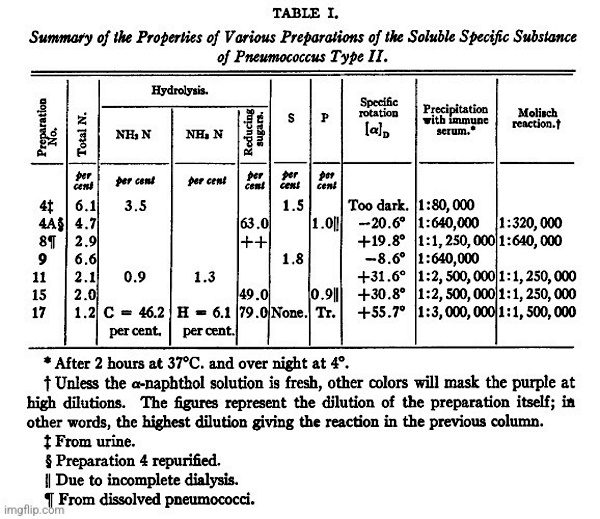
“Table I represents a summary of the reactions of some of the earlier preparations worked with, as well as the later ones. Preparation 4 was obtained from the urine of a patient with a Type II pneumococcus infection, while No. 8 was obtained from an antiforrain solution of the pnemnococci. In both of these cases, as well as in Nos. 9, 11, and 15, the method of purification given above had not been fully worked out.
Attempts to stimulate antibody production by the immunization of animals with the purified substance yielded negative results.
Discussion.
While it has long been known that the capsular material of many microorganisms consists, at least in part, of carbohydrates, any connection between this carbohydrate material and the specificity relationships of bacteria appears to have remained unsuspected. While it cannot be said that the present work establishes this relationship, it certainly points in this direction. Evidence in favor of the probable carbohydrate nature of the soluble specific substance is the increase in specific activity with reduction of the nitrogen content, the increase in optical rotation with increase in specific activity, the parallelism between the Molisch reaction and specific activity, the high yield of reducing sugars on hydrolysis, and the actual isolation of glucosazone from a small quantity of the material. The small amounts of substance available up to the present have hindered the solution of the problem, and it is hoped that efforts at further purification of the soluble specific substance, now in progress with larger amounts of material, will definitely settle the question.
Summary.
-
- A method is given for the concentration and purification of the soluble specific substance of the pneumococcus.
- The material obtained by this method is shown to consist mainly of a carbohydrate which appears to be a polysaccharide built up of glucose molecules.
- Whether the soluble specific substance is actually the polysaccharide, or occurs merely associated with it, is still undecided, although the evidence points in the direction of the former possibility.”

Heidelberger’s original 1923 paper can hardly be claimed to be the slam-dunk proof that bacteria antigens are carbohydrates. For starters, Heidelberger admitted that he was unsure if the presumed “antigen” substance was a carbohydrate or if it was merely associated with it. Even more importantly, he could not produce any antibody response upon injecting his presumed antigen into animals. This would indicate that the substance was not an antigen whatsoever as antigens are specifically defined as “a toxin or other foreign substance which induces an immune response in the body, especially the production of antibodies.” Thus, it seems rather odd to assume antibodies are proteins based off of this work, but assume they did:
Michael Heidelberger 1888–1991
“Since the pneumococcal capsular antigen was a polysaccharide, and antibodies were thought to be proteins, Heidelberger realized that by measuring the amount of protein in specific precipitates made with the capsular antigen he could determine their antibody content. Together with Forrest Kendall, who had joined the Heidelberger lab, the protein content of immune precipitates was determined by measuring total nitrogen, using the Kjeldahl procedure that came to be the hallmark of laboratories carrying out Heidelberger-type quantitative immunochemistry.”
Since they assumed the pneumococci bacteria was a polysaccharide, that meant any nitrogen left over was the antibody content. Based on the 1923 paper, this seems to be a rather falicious premise to build from. In any case, Heidelberger carried on with his assumption and it can be seen by this second paper from 1929 how Heidelberger came to his conclusion using the precipitin test and mathematics as proof that antibodies exist. I edited out the long mathematical sections with his equations so if you are interested in Heidelberger showing his work, I recommend reading the full paper. Highlights below:
A Quantitative Study of the Precipitin Reaction Between Type III Pneumococcus Polysaccharide and Purified Homologous Antibody*
“Of all the reactions of immunity the precipitin test is perhaps the most dramatic and striking. While other immune reactions are more delicate, the precipitin test is among the most specific and least subject to errors and technical difficulties. Attempts at its quantitative interpretation and explanation have been hampered either by the difficulty of finding suitable analytical methods or by the failure to separate the reacting substances from closely related, non-specific materials with which they are normally associated.
With the aid of recent work it has been found possible to avoid these difficulties to some extent. The isolation of bacterial polysaccharides which precipitate antisera specifically and possess the properties of haptens has not only afforded one of the components of a precipitin reaction in a state of comparative purity, but has greatly simplified the analytical problem. Since many of these polysaccharides contain no nitrogen, and antibodies presumably are nitrogenous, the latter may be determined in the presence of any amount of the specific carbohydrate. Moreover, Felton’s method for the separation of pneumococcus antibodies from horse serum not only permits the isolation of a high proportion of the precipitin, freed from at least 90 percent of the serum proteins and much of the serum lipoid, but is also applicable on a sufficiently large scale to furnish the amounts of antibody solution needed to make quantitative work possible. It is realized that antibody solutions of this type do not contain pure antibodies–indeed, only 40 to 50 percent of the nitrogen is specifically precipitable–but since so small a proportion of the original serum protein remains with the antibody a far-reaching purification actually has been effected. It should thus be possible with the aid of antibodies purified by Felton’s method to obtain data of a preliminary character which should point toward the mechanism of the reaction. The present paper is concerned with such data obtained in a quantitative study of the precipitin reaction between the soluble specific substance of Type III pneumococcus and Type III pneumococcus antibody solution.
Experimental
1. Materials and Methods.–a. Solutions of Soluble Specific Substance, Type Ill
Pneumococcus.–The soluble specific substance of Type III pneumococcus used was kindly supplied by Drs. O. T. Avery and W. F. Goebel of The Rockefeller Institute for Medical Research. It was ash-free, contained 0.04 percent of nitrogen, and showed a/d = -32 °. A weighed amount of anhydrous substance was suspended in 0.9 percent saline, dissolved with the aid of 0.1 normal sodium hydroxide, and the solution was diluted with saline, adjusted to pH 7.6 and made up to volume with saline to yield a 1 percent solution. This was sterilized in the autoclave and used as a stock solution for making up other dilutions. These were prepared with sterile saline under aseptic precautions, and were kept in the ice-box.
b. Type III Pneumococcus Antibody Solution.–The antibody solutions used were prepared essentially according to Felton’s procedure (loc. cit.) from Type III antipneumococcus horse serum containing no preservative and supplied by the New York State Department of Health through the courtesy of Dr. A. B. Wadsworth and Dr. Mary B. Kirkbride. 100 to 200 cc. of serum were stirred slowly into 20 volumes of ice-cold water containing 9.5 cc. of molar potassium dihydrogen phosphate and 0.5 cc. of molar dipotassium hydrogen phosPhate per liter. The final pH varied from 5.6 to 6.3. After standing over night in the cold the supernatant was decanted and the precipitate was centrifuged off in the cold and dissolved in a volume of chilled 0.9 percent saline equal to that of the serum taken. 0.1 normal hydrochloric acid was then added until a precipitate no longer formed on dilution of a test portion with two volumes of water, after which 0.1 normal sodium hydroxide solution was added until a slight precipitate again formed on dilution. In general, 5 cc. of acid and 1.5 cc. of alkali per 100 cc. of serum were satisfactory, although as Felton emphasizes, different lots vary and no absolutely definite procedure can be given. In the present work the process of purification was followed either by testing the agglutinating power of the fractions against a heat-killed Type III pneumococcus vaccine, or by the precipitin reaction, or by both methods. After addition of the alkali the opalescent solution was diluted with 2 volumes of water and centrifuged in the cold. The almost inactive precipitate was discarded and the supernatant poured into 6.7 volumes of the chilled buffer solution previously used, (equivalent to 20 times the volume of saline employed), also adding enough 0.1 normal sodium hydroxide to neutralize the remaining acid. The resulting precipitate was collected and dissolved in a volume of 0.9 percent saline equal to that of the serum taken, and the pH was adjusted to 7.6. The solution was sterilized by passage through a Berkefeld N grade filter which previously had been washed with saline containing a drop of normal sodium hydroxide, followed by saline alone.
Antibody solutions prepared in this way were found to be rather unstable under the usual conditions of the precipitin test, and it therefore was necessary to subject them to a preliminary “ageing” treatment in order that control solutions might be relied upon to remain clear. This consisted in immersing the solution in a water bath at 37 ° for 2 hours, letting stand in the ice-box over night, centrifuging off the precipitate which usually formed, readjusting the pH if necessary, and filtering through a Berkefeld candle prepared as above. This treatment was repeated as many times as necessary, but the solutions usually remained clear after the second incubation at 37 °. Much time was lost and very inconstant results were obtained until “ageing” was resorted to.
The relative antibody content of the resulting solutions was estimated by determining the agglutination titer against a single heat-killed Type III pneumococcus suspension.
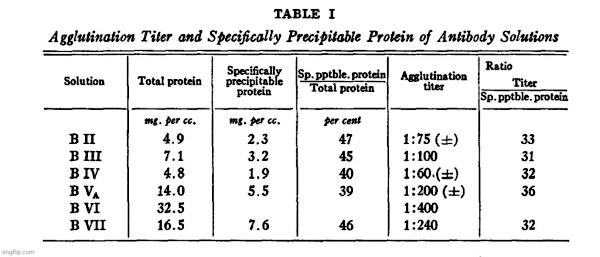
It will be seen from Table I that the agglutination titer and the
maximum amount of protein precipitable by the type III polysaccharide ([total N–N in supernatant] X 6.25) are approximately proportional. The latter may therefore be taken as a more definite, though not necessarily more accurate, measure of the actual antibody content of the solutions.
It is also evident that the antibody in all of these solutions has been purified to approximately the same extent, since the ratios of protein precipitable by SSS III to total protein are not very different.”
Discussion
“For purposes of discussion it will be assumed with Felton (lot. cir.) that antibody is ,modified protein, and that, in order to provide a uniform method of measurement, it may be expressed as nitrogen precipitable by specific polysaccharide, multiplied by 6.25. Since only relative values are under consideration, the actual magnitude of the factor used is of little significance so long as it be used throughout. Moreover, Table I shows a correspondence between this measure of antibody content and the agglutination titer, so that its use as a relative measure is independent of the nature of Type III pneumococcus antibodies.
doi: 10.1084/jem.50.6.809.
In Summary:
- Michael Heidelberger teamed up with Oswald Avery to characterize a “soluble specific substance” found in pneumococcal bacteria that fell out of solution when incubated with type-specific antisera
- When it was virtually nitrogen-free, recalled Heidelberger in a 1979 article, Avery ventured a guess: “Could it be a carbohydrate?”
- Chemical analysis confirmed its sugary character, and subsequent studies of other pneumococcal serotypes revealed that each bacterial capsule had a distinct polysaccharide signature
- It was this signature that dictated the serological specificity of the organism
- Their results were met with considerable skepticism, as it was then thought that only proteins could incite a specific immune response
- According to polysaccharide-based vaccine specialist Emil Gotschlich: “Nobody believed it. It took them a lot of effort to convince people that the polysaccharide was the immunoreactive component.”
- Heidelberger and Avery’s discovery came at a time when antibodies were regarded—by those who believed they existed at all—as mysterious substances that floated around in serum
- “It appeared to me that there was a crying need to determine the true nature of antibodies,” wrote Heidelberger in 1979, “and that until this was done there could be no end to the polemics and uncertainties that were plaguing immunology”
- Heidelberger and his postdoctoral fellow Forrest Kendall later quantitated the precipitin reaction, bringing much-needed mathematics to the study of antibody–antigen interactions and lifting antibodies even further out of the realm of the mysterious

- In 1917 Dochez and Avery showed that whenever pneumococci are grown in fluid media, there is present in the cultural fluid a substance which precipitates specifically in antipneumococcus serum of the homologous type
- It was assumed that the formation of this soluble specific material by pneumococci on growth in vitro suggested the probability of an analogous substance being formed on growth of the organism in the animal body
- Examination of the urine of patients with pneumococci showed the substance in only approximately 2/3rds of the samples
- Zinsser and Parker found similar substances with other bacteria and believe that the substances are the same as that of the pneumococci
- In spite of the fact that these “residue antigens” are precipitable by homologous sera produced by immunization with the whole bacteria, Zinsser and Parker failed to produce antibodies in animals by injecting the residues.
- The process for the isolation of the soluble specific substance consisted in:
-
- Concentration of the broth
- Precipitation with alcohol
- Repeated re-solution and reprecipitation
- A careful series of fractional precipitations with alcohol or acetone after acidification of the solution with acetic acid
- Repeated fractional precipitation with ammonium sulfate and dialysis of the aqueous solution of the active fraction
- For a complete step-by-step breakdown of the numerous chemical-altering procedures done to the sample, see the highlighted tan section of the paper provided above
- Even with the numerous “purification” steps, the obtained soluble specific substance formed an almost colorless varnish-like mass
- The residual material for which no claim of purity was made, as efforts at its further purification were still under way, contained, on the ash-free basis, 1.2 percent of nitrogen.
- It was considered essentially a polysaccharide
- The method of purification given had not been fully worked out for many of the preparations
- Attempts to stimulate antibody production by the immunization of animals with the purified substance yielded negative results
- While it had long been known that the capsular material of many microorganisms consists, at least in part, of carbohydrates, any connection between this carbohydrate material and the specificity relationships of bacteria remained unsuspected
- While it could not be said that their work established this relationship, they felt it certainly pointed in that direction
- The small amounts of substance available hindered the solution of the problem, and it was hoped that efforts at further purification of the soluble specific substance with larger amounts of material would definitely settle the question
- Whether the soluble specific substance is actually the polysaccharide, or occurs merely associated with it, was left undecided

- Heidelberger acknowledged that the precipitin test he used during this experiment has 2 drawbacks:
-
- Quantitative interpretation/explanation is difficult due to lack of a suitable analytical method
- Failure to separate out the reacting substances from non-specific material which these substances are closely related to and associated with
- He stated that it was possible to avoid these failures to some extent
- It is presumed that antibodies are nitrogenous
- Only 90% of the precipitin can be freed from serum proteins and “much” of the lipoid
- Heidelberger admitted that these are not pure antibodies and that only 40-50% of nitrogen is precipitable while small amounts of serum remain
- The antibody solutions used were prepared essentially according to Felton’s procedure from Type III antipneumococcus horse serum containing no preservative and supplied by the New York State Department of Health through the courtesy of Dr. A. B. Wadsworth and Dr. Mary B. Kirkbride
-
- 100 to 200 cc. of serum were stirred slowly into 20 volumes of ice-cold water containing 9.5 cc. of molar potassium dihydrogen phosphate and 0.5 cc. of molar dipotassium hydrogen phosphate per liter
- The final pH varied from 5.6 to 6.3
- After standing over night in the cold the supernatant was decanted and the precipitate was centrifuged off in the cold and dissolved in a volume of chilled 0.9 percent saline equal to that of the serum taken
- 0.1 normal hydrochloric acid was then added until a precipitate no longer formed on dilution of a test portion with two volumes of water, after which 0.1 normal sodium hydroxide solution was added until a slight precipitate again formed on dilution
- In general, 5 cc. of acid and 1.5 cc. of alkali per 100 cc. of serum were satisfactory, although as Felton emphasized, different lots vary and no absolutely definite procedure can be given
- After addition of the alkali the opalescent solution was diluted with 2 volumes of water and centrifuged in the cold
- The almost inactive precipitate was discarded and the supernatant poured into 6.7 volumes of the chilled buffer solution previously used, (equivalent to 20 times the volume of saline employed), also adding enough 0.1 normal sodium hydroxide to neutralize the remaining acid
- The resulting precipitate was collected and dissolved in a volume of 0.9 percent saline equal to that of the serum taken, and the pH was adjusted to 7.6
- The solution was sterilized by passage through a Berkefeld N grade filter which previously had been washed with saline containing a drop of normal sodium hydroxide, followed by saline alone
- Antibodies were found to be unstable during testing so they were put through preliminary “ageing” processes as many times as needed until they got the result they wanted
- Much time was lost and very inconstant results were obtained until “ageing” was resorted to.
- The relative antibody content of the resulting solutions was estimated by determining the agglutination titer against a single heat-killed Type III pneumococcus suspension
- For purposes of discussion it was assumed with Felton that antibody is modified protein, and that, in order to provide a uniform method of measurement, it may be expressed as nitrogen precipitable by specific polysaccharide, multiplied by 6.25
- There is no need to spend any more time on the rest of Heidelberger’s paper as he admitted he assumed antibodies were protein and could be expressed as nitrogen thus he did not prove anything
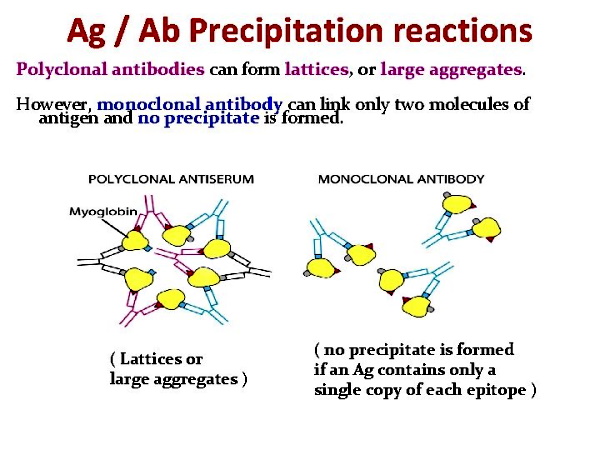
It is rather obvious that many assumptions were made about a substance (antibodies) for which the researchers could not see. Michael Heidelberger assumed that antibodies are modified proteins and nitrogenous. He assumed that it may be expressed as nitrogen precipitable by specific polysaccharide, multiplied by 6.25. He assumed that the failure of the precipitin test to separate out the reacting substances from non-specific material which these substances are closely related to and associated with could be somewhat avoided to some extent. He assumed that his earlier work with the pneumococcus bacteria was accurate and that he had proved the antigen component was a carbohydrate even though he was unable to produce any antibody response upon immunizing animals using his supposed antigen. Maybe this lack of any antibody response to his “antigen” has to do with the fact that, according to the WHO, the pneumococcus bacteria is regularly found in healthy people?
“Infection is acquired mainly through pneumococci contained in respiratory droplets. There are many healthy, asymptomatic carriers of the bacteria but no animal reservoir or insect vector.”
https://www.who.int/ith/diseases/pneumococcal/en/
https://web.archive.org/web/20200818101511/https://www.who.int/ith/diseases/pneumococcal/en/
If an antigen is a toxin or foreign substance which produces an immune response creating antibodies, the pneumococci bacteria doesn’t meet that definition at all. If it isn’t an antigen, then the pneumococcus “antigen” would not be carbohydrates as described in Heidelberger’s 1923 paper. This would mean that Heidelberger’s 1929 paper measuring any of the remaining protein content, calculating the amount, and claiming the resulting protein mass as antibodies is essentially meaningless. Can you see the problem with assuming things to be true without ever proving this to be the case?
The conclusions drawn by Heidelberger were born out of chemistry experiments and reactions using the precipitin test which have no bearing on reality while using mathematical equations attempting to quantify the unquantifiable. Whether or not these indirect experiments and assumptions provide proof that antibodies exist and are proteins, I leave up to the reader. However, keep in mind that no antibodies had ever been seen nor proven to exist by proper purification and isolation up to that time and that still holds true to date. This work is based off of theoretical explanations of immunity for which nothing could be observed. Heidelberger’s indirect chemical reactions and equations provided no direct evidence for the existence of anything other than non-specific precipitate.
Connect with Mike Stone, ViroLIEgy

Truth Comes to Light highlights writers and video creators who ask the difficult questions while sharing their unique insights and visions.
Everything posted on this site is done in the spirit of conversation. Please do your own research and trust yourself when reading and giving consideration to anything that appears here or anywhere else.










