
CDC Drops Quarantine, Distancing Recommendations, as 1.3 Million COVID Vaccine Injuries Reported to VAERS
VAERS data released Friday by the Centers for Disease Control and Prevention show 1,379,438 reports of adverse events from all age groups following COVID-19 vaccines, including 30,162 deaths and 251,075 serious injuries between Dec. 14, 2020, and Aug. 5, 2022.
by Megan Redshaw, The Defender
August 12, 2022
Editor’s note: In previous VAERS weekly updates, The Defender focused exclusively on U.S. reports in the sections where reports are broken out by age groups and types of adverse events. However, excluding foreign reports from these categories excludes thousands of vaccine injuries reported to the system, so those sections now include all — U.S. and foreign combined — reports submitted to VAERS in the categories indicated.
The Centers for Disease Control and Prevention (CDC) today released new data showing a total of 1,379,438 reports of adverse events following COVID-19 vaccines were submitted between Dec. 14, 2020, and Aug. 5, 2022, to the Vaccine Adverse Event Reporting System (VAERS). That’s an increase of 7,964 adverse events over the previous week.
VAERS is the primary government-funded system for reporting adverse vaccine reactions in the U.S.
The data included a total of 30,162 reports of deaths — an increase of 181 over the previous week — and 251,075 serious injuries, including deaths, during the same time period — up 1,959 compared with the previous week.
Of the 30,162 reported deaths, 19,462 cases are attributed to Pfizer’s COVID-19 vaccine, 8,038 cases to Moderna, 2,613 cases to Johnson & Johnson (J&J) and no cases yet reported for Novavax.
Excluding “foreign reports” to VAERS, 854,084 adverse events, including 13,972 deaths and 87,488 serious injuries, were reported in the U.S. between Dec. 14, 2020, and Aug. 5, 2022.
Foreign reports are reports foreign subsidiaries send to U.S. vaccine manufacturers. Under U.S. Food and Drug Administration (FDA) regulations, if a manufacturer is notified of a foreign case report that describes an event that is both serious and does not appear on the product’s labeling, the manufacturer is required to submit the report to VAERS.
Of the 13,972 U.S. deaths reported as of Aug. 5, 7% occurred within 24 hours of vaccination, 15% occurred within 48 hours of vaccination and 54% occurred in people who experienced an onset of symptoms within 48 hours of being vaccinated.
In the U.S., 604 million COVID-19 vaccine doses had been administered as of Aug. 3, including 357 million doses of Pfizer, 228 million doses of Moderna and 19 million doses of Johnson & Johnson (J&J).
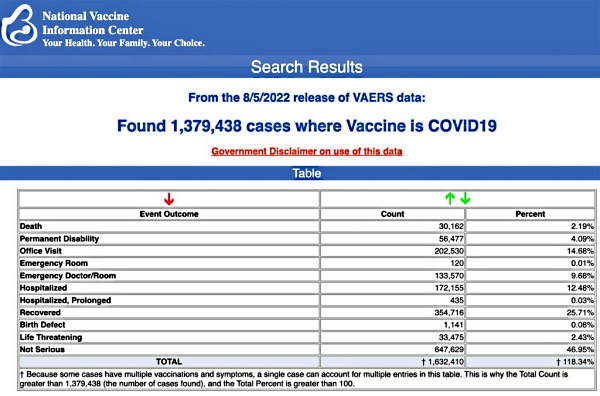
Every Friday, VAERS publishes vaccine injury reports received as of a specified date. Reports submitted to VAERS require further investigation before a causal relationship can be confirmed.
Historically, VAERS has been shown to report only 1% of actual vaccine adverse events.
VAERS data from Dec. 14, 2020, to Aug. 5, 2022, for 6-month-olds to 5-year-olds show:
- 2,975 adverse events, including 140 cases rated as serious and 5 reported deaths.
- 4 reports of myocarditis and pericarditis (heart inflammation).
The CDC uses a narrowed case definition of “myocarditis,” which excludes cases of cardiac arrest, ischemic strokes and deaths due to heart problems that occur before one has the chance to go to the emergency department. - 20 reports of blood clotting disorders.
- 38 reports of seizures.
VAERS data from Dec. 14, 2020, to Aug. 5, 2022, for 5- to 11-year-olds show:
- 13,364 adverse events, including 620 rated as serious and 22 reported deaths.
- 45 reports of myocarditis and pericarditis.
- 64 reports of blood clotting disorders.
- 169 reports of seizures.
VAERS data from Dec. 14, 2020, to Aug. 5, 2022, for 12- to 17-year-olds show:
- 32,945 adverse events, including 4,189 rated as serious and 118 reported deaths.
According to the CDC, “VAERS data available to the public include only the initial report data to VAERS. Updated data which contains data from medical records and corrections reported during follow up are used by the government for analysis. However, for numerous reasons including data consistency, these amended data are not available to the public.” - 268 reports of anaphylaxis among 12- to 17-year-olds where the reaction was life-threatening, required treatment or resulted in death — with 94% of cases attributed to Pfizer’s vaccine.
- 1,304 reports of myocarditis and pericarditis with 650 cases attributed to Pfizer’s vaccine.
- 298 reports of blood clotting disorders with 275 cases attributed to Pfizer.
- 26 cases of postural orthostatic tachycardia syndrome (POTS) with all cases attributed to Pfizer’s vaccine.
VAERS data from Dec. 14, 2020, to Aug. 5, 2022, for all age groups combined, show:
- 20% of deaths were related to cardiac disorders.
- 55% of those who died were male, 41% were female and the remaining death reports did not include the gender of the deceased.
- The average age of death was 73.
- As of Aug. 5, 12,010 pregnant women reported adverse events related to COVID-19 vaccines, including 4,801 reports of miscarriage or premature birth.
- Of the 16,124 cases of Bell’s Palsy reported, 75% were attributed to Pfizer vaccinations, 23% to Moderna and 4% to J&J.
- 2,881 reports of Guillain-Barré syndrome, with 66% of cases attributed to Pfizer, 20% to Moderna and 17% to J&J.
- 9,884 reports of anaphylaxis where the reaction was life-threatening, required treatment or resulted in death.
- 4,663 reports of myocardial infarction.
- 42,180 reports of blood-clotting disorders in the U.S. Of those, 28,921 reports were attributed to Pfizer, 9,445 reports to Moderna and 3,754 reports to J&J.
- 23,535 cases of myocarditis and pericarditis with 17,890 cases attributed to Pfizer, 5,226 cases to Moderna and 399 cases to J&J.
- 64 cases of Creutzfeldt-Jakob disease with 51 cases attributed to Pfizer, 12 cases to Moderna and 1 case to J&J.
- 493 cases of POTS with 375 cases attributed to Pfizer, 109 cases to Moderna and 21 cases to J&J.
Children’s Health Defense (CHD) asks anyone who has experienced an adverse reaction, to any vaccine, to file a report following these three steps.
New CDC COVID guidance ditches distinctions between vaccinated and unvaccinated
The CDC on Thursday issued sweeping new recommendations as part of the agency’s efforts to overhaul its COVID-19 guidance.
“This guidance acknowledges that the pandemic is not over, but also helps us move to a point where COVID-19 no longer severely disrupts our daily lives,” the CDC’s Greta Massetti said in a press release.
Here are the biggest changes to the CDC’s guidance:
- Unvaccinated people now have the same guidance as vaccinated people.
- Those who are exposed to the virus are no longer required to quarantine regardless of vaccination status.
- Students may stay in class even if they’ve been exposed to COVID-19.
- Six-foot social distancing is no longer recommended.
- Contact tracing and routine surveillance testing of symptomatic people are no longer recommended in most settings.
According to The New York Times, the CDC has been working for months on the new guidance which builds on previous guidance issued in February that reduced isolation times for those who get COVID-19.
The agency said it is making changes to its guidance now because “vaccination and prior infections have granted many Americans some degree of protection against the virus, and treatments, vaccines and boosters are available to reduce the risk of severe illness.”
According to The National Law Review:
“The CDC’s focus on individual responsibility, the removal of distinctions between vaccinated and unvaccinated, the removal of quarantine recommendations and the discussion of mask wearing as an individual responsibility are good news for employers who are considering relaxing COVID-19 workplace requirements.
“This likely will not be the last we hear from the CDC on this topic. Indeed, the CDC stated that it intends to issue more specific guidance for settings such as healthcare, congregate living, and travel.”
Pfizer vaccine efficacy in teens wanes 27 days after second dose
A study published Aug. 8 in The Lancet showed the effectiveness of the Pfizer-BioNTech COVID-19 vaccine against symptomatic infection among adolescents “rapidly declined over time,” waning from just 27 days after the second dose.
Researchers analyzed data from 503,776 COVID-19 tests of 2,948,538 adolescents — ages 12-17 — in Brazil from Sept. 2, 2021, to April 19, 2022, and 127,168 tests of 404,673 adolescents in Scotland from Aug. 6, 2021, to April 19, 2022.
The study showed vaccine efficacy began to decline 27 days after the second dose for both countries, plummeting to 5.9% (95% CI 2.2–9.4) in Brazil and dropping to 50.6% (95% CI 42.7–57.4) in Scotland at 98 days after adolescents received the second dose.
While protection against symptomatic COVID-19 dropped dramatically in both countries less than one month after the second dose, protection against severe illness — defined as hospitalization or death within 28 days — remained above 80% in Brazil from 28 days to 98 days and beyond.
The authors sought to assess protection against severe illness in Scotland but were unable to do so because so few cases of severe COVID-19 in adolescents in Scotland were reported during the time of the study.
The authors concluded that “two doses are insufficient to sustain protection against symptomatic disease” in adolescents and recommended more research be done on the need for booster doses.
‘Stunning’ link between Pfizer vaccine and myocarditis in teens, study shows
A preprint study conducted during Thailand’s national COVID-19 vaccination campaign showed what one physician described as a “stunning” association between myocarditis and the Pfizer-BioNTech vaccine.
The study analyzed 301 participants ages 13-18 who were healthy and without abnormal symptoms after receiving their first vaccine dose. Participants with a history of cardiomyopathy, tuberculous pericarditis or constrictive pericarditis and severe allergic reaction to the COVID-19 vaccine were excluded from the study.
Researchers found that 18% of the 301 teens analyzed had an abnormal electrocardiogram, or EKG after receiving their second dose of Pfizer, 3.5% of males developed myopericarditis or subclinical myocarditis, two were hospitalized and one was admitted to the ICU for heart problems.
Cardiovascular adverse events observed during the study included tachycardia (7.64%), shortness of breath (6.64%), palpitation (4.32%), chest pain (4.32%) and hypertension (3.99%).
Fifty-four adolescents had abnormal electrocardiograms after vaccination, three patients had minimal pericardial effusion with findings compatible with subacute myopericarditis and six patients experienced mitral valve prolapse.
All patients were male and had abnormal electrocardiograms, particularly sinus tachycardia. Researchers said the clinical course was mild in all cases.
Military using ‘Comirnaty’ vaccine produced at facility not approved by FDA
In an exclusive interview with The Defender, a U.S. Coast Guard (USCG) service member alleged the U.S. Department of Defense (DOD) is administering COVID-19 vaccines from vials of Pfizer’s Comirnaty-labeled vaccines that are not produced in a facility approved by the FDA.
Lt. Chad R. Coppin, in a July 30 declaration submitted to Sen. Ron Johnson (R-Wis.) under penalty of perjury, detailed his personal investigation into the availability and origin of Comirnaty-labeled COVID-19 vaccine vials at U.S. military facilities.
Coppin relayed his concerns in an interview with The Defender, as did Holly Freincle, the wife of a U.S. military service member stationed at Fort Detrick, Maryland, who corroborated Coppin’s claims that Comirnaty-labeled vaccine vials are appearing at military service facilities.
Until now, the DOD has claimed the Pfizer-BioNTech COVID-19 vaccine, administered under Emergency Use Authorization, is “interchangeable” with the fully licensed Pfizer Comirnaty vaccine — which until recently, was said to be unavailable at military facilities.
In his July 30 declaration, Coppin, who has served with the USCG since March 2002, reported that after a long period of unavailability, the “Comirnaty” vaccine began to appear at U.S. military facilities in June.
Connect with Children’s Health Defense
©August 2022 Children’s Health Defense, Inc. This work is reproduced and distributed with the permission of Children’s Health Defense, Inc. Want to learn more from Children’s Health Defense? Sign up for free news and updates from Robert F. Kennedy, Jr. and the Children’s Health Defense. Your donation will help to support us in our efforts.
cover image credit: geralt

Truth Comes to Light highlights writers and video creators who ask the difficult questions while sharing their unique insights and visions.
Everything posted on this site is done in the spirit of conversation. Please do your own research and trust yourself when reading and giving consideration to anything that appears here or anywhere else.










