The Exosome Concept
by Mike Stone, ViroLIEgy
June 27, 2022
Although originally ignored as cell debris, it is increasingly evident that exosome release is regulated and occurs via an energy-dependent pathway. Exosomes are believed to ferry proteins, mRNA, and miRNA cargos through the bloodstream and other body fluids, shielding them from enzymatic degradation—a process that some retroviruses may hijack to travel beneath the immune system’s radar.”
https://www.ahajournals.org/doi/10.1161/circresaha.113.300636
During the past two plus years, exosomes have become a hotly discussed topic among those questioning the “virus” lie. This is primarily due to Dr. Andrew Kaufman bringing them to prominence in his original video questioning the existence of “SARS-COV-2.” Even though these entities have been known about for the last 40 years, many people, including myself, had either never heard of these particles or had not paid much attention to them. Dr. Kaufman did a great job showcasing how the particles known as exosomes are the exact same particles associated with “SARS-COV-2” as seen in EM images. They were just given different names and functions.
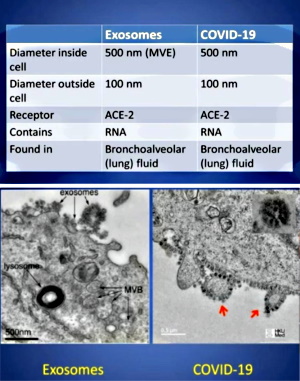
With this new spotlight on exosomes, many people who had begun questioning the “viral” narrative replaced the “virus” concept with the exosome concept. It appeared to them that this was just a case of mistaken identity. The harmful pathogenic “viruses” were being misidentified this whole time and were in fact just beneficial exosomes carrying information between the cells.
While they rightfully questioned the evidence for the existence of “viruses” and also understood that the same particles are used as representation for both “viruses” and exosomes, these people latched on to the belief that the evidence for the existence of exosomes somehow passed the scientific smell test. They believe that, unlike “viruses,” exosomes have been purified, isolated, characterized, and that their functions have been scientifically proven. However, nothing could be further from the truth.
Exosomes/”Viruses:” Same Particles, Same Faulty “Science”
I have written many articles on the inability to completely purify and isolate exosomes from “viruses” and other particles of similar size and density. This is a fundamental problem for exosome and “viral” research as without being able to separate the particles assumed to be exosomes from those claimed to be “viruses,” there is no way to be able to study either independently, distinguish them from any of the other particles, nor to characterize the particles properly. This problem was expressed in the article Extracellular Vesicles and Viruses – Two Sides of the Same Coin?:
“How can we be sure that we are isolating and quantifying extracellular vesicles rather than enveloped viruses present in the sample? Equally, how can viral researchers know that they are not detecting similarly sized non-viral vesicles or empty vectors during vaccine production?”
Somehow, people are under the impression that exosomes can be completely separated from everything else. While it is true that exosome researchers will put their samples through greater purification steps than those seen in “virus” research, it is admitted regularly by these researchers that complete separation can not be achieved by the current methods, even with the “gold standard” ultracentrifugation:
“Unless more specifically defined, it is currently virtually impossible to specifically separate and identify EVs that carry viral proteins, host proteins, and viral genomic elements from enveloped viral particles that carry the same molecules.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4995926/
“Nowadays, it is an almost impossible mission to separate EVs and viruses by means of canonical vesicle isolation methods, such as differential ultracentrifugation, because they are frequently co-pelleted due to their similar dimension [56,57]. To overcome this problem, different studies have proposed the separation of EVs from virus particles by exploiting their different migration velocity in a density gradient or using the presence of specific markers that distinguish viruses from EVs [56,58,59]. However, to date, a reliable method that can actually guarantee a complete separation does not exist.”
Click to access viruses-12-00571.pdf
“Since it is near impossible to separate EV from virions by biochemical methods, the absence of EV is typically demonstrated by the absence of EV protein markers.”
Even if the researchers combine purification methods, they are unable to entirely separate the particles claimed to be exosomes from everything else. If they are unable to get the particles they claim are exosomes away from “viruses” and other similar particles of the same size, density, and morphology, this would mean any electron microscope image of the particles in question are useless as they could potentially be anything, as I have shown in numerous articles discussing these problematic images. Yet an even bigger problem is that due to the nature of EM, the particles called exosomes can only be seen in a dead state. As we can not peer into the body to see these particles at work, their functioning can not be observed. What they do or if they even float around in the body as presented is anyone’s best guess, as pointed out in the opening quote to this article as well as in numerous other sources:
“Exosomes, once thought to be biomarkers of a diseased state are now thought to be biologically active and some of the paracrine effects of stem cell therapy.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5161232/
“First, exosomes are thought to be a medium for cell communication and intercellular macromolecular transport.”
“First, they are thought to provide a means of intercellular communication and of transmission of macromolecules between cells. Second, in the past decade, exosomes have been attributed roles in the spread of proteins, lipids, mRNA, miRNA and DNA and as contributing factors in the development of several diseases. And third, they have been proposed to be useful vectors for drugs because they are composed of cell membranes, rather than synthetic polymers, and as such are better tolerated by the host.”
“Yet despite 20 years of research, the very basics of exosome biology are in their infancy and we know little of the part they play in normal cellular physiology.”
https://bmcbiol.biomedcentral.com/articles/10.1186/s12915-016-0268-z
As can be seen from the above sources, the role that the particles claimed to be exosomes play in the human body is thought to be one of intercellular communication and transport. They have been attributed roles and have had functions proposed. However, even after decades of research, researchers still do not know what these particles do. They only have guesses, assumptions, and hypotheses. In fact, the particles now called exosomes were originally regarded as nothing more than cellular debris created through the process of cell death known as apoptosis:
“They were initially thought to be “cellular dust” or served as a mechanism by which cells actively dispose of their own waste [3].”
https://www.sciencedirect.com/science/article/pii/S0753332220304297
What is Apoptosis?
When cells die, they go into a programmed cell death known as apoptosis where the cell begins to break apart and collapse which then releases tiny particles of cellular debris and waste. This process is separated into 5 main steps:
Major steps of apoptosis:
1. Cell shrinks
2. Cell fragments
3. Cytoskeleton collapses
4. Nuclear envelope disassembles
5. Cells release apoptotic bodies
https://www.cipf.es/science/core-facilities/electron-microscopy
The last step listed above is the release of what are called apoptotic bodies. What are apoptotic bodies?
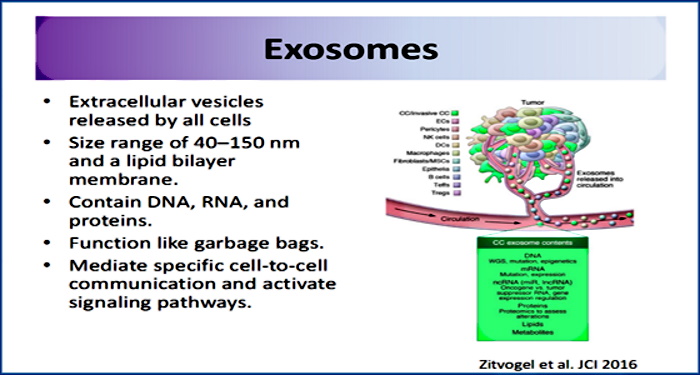
“Apoptotic bodies, “little sealed sacs” containing information and substances from dying cells, were previously regarded as garbage bags until they were discovered to be capable of delivering useful materials to healthy recipient cells (e.g., autoantigens) [23].”
The particles called apoptotic bodies, which can range in size anywhere from 50 to 5000 nm, were considered “garbage bags” containing information from dying cells until they were “discovered” to carry useful materials to healthy cells. Where have I seen this description before?
Exosomes: Revisiting their role as “garbage bags”
“Fifteen years ago, we proposed that one physiological function of exosomes could be a clearance process, whereby exosomes would serve as a quality control system to verify the “recyclability” of membrane molecules.”
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7168913/
“At first exosomes were thought to function as “cellular garbage bags”, but now these nano-sized extracellular vesicles are being studied for their role in progression and metastasis.”
https://tcr.amegroups.com/article/view/14924/html
“Exosomes were initially thought to serve simply as “garbage bags” for cells to get rid of unwanted constituents.”
https://www.hindawi.com/journals/tswj/2015/657086/
This description of tiny particles which were considered garbage bags that also transport information and cargo between cells can be applied to both exosomes and apoptotic bodies. In fairness, these particles both fall under the larger umbrella term of extracellular vesicles. However, there is much more blurring the lines between these particles other than their definitions. It is stated that they both fall into the same size range (along with ectosomes and “viruses”) and that understanding and completely distinguishing these entities based on their differences has been overlooked:
“There are other types of microvesicle, including apoptotic bodies and ectosomes, which are derived from cells undergoing apoptosis and plasma membrane shedding, respectively. Although apoptotic bodies, ectosomes and exosomes are all roughly the same size (typically 40–100 nm) and all also contain ‘gulps’ of cytosol, they are different species of vesicles and understanding differences between them is of paramount importance but has too often been overlooked.”
https://bmcbiol.biomedcentral.com/articles/10.1186/s12915-016-0268-z
This blurring of the line does not stop there. In an article from January 2020, it is discussed that exosomes are in fact released by apoptosis thus showing that exosomes and apoptotic bodies are both created from the same cell death process. This is further evidence that they are in fact the same exact particles just at different stages and given different names and functions:
“Apoptosis, a type of programmed cell death that plays a key role in both healthy and pathological conditions, releases extracellular vesicles such as apoptotic bodies and microvesicles, but exosome release due to apoptosis is not yet commonly accepted. Here, the reports demonstrating the presence of apoptotic exosomes and their roles in inflammation and immune responses are summarized, together with a general summary of apoptosis and extracellular vesicles. In conclusion, apoptosis is not just a ‘silent’ type of cell death but an active form of communication from dying cells to live cells through exosomes.”
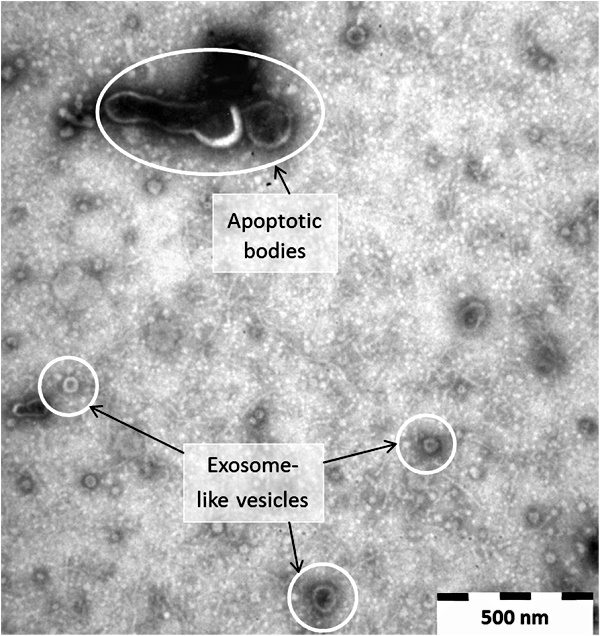
Why is this connection between apoptotic bodies and exosomes important? As both have been coined garbage bags and considered cellular debris/waste that occur during cell death, it can be seen that these particles, if they represent anything at all, are just waste material from dying cells which serve no purpose whatsoever. This makes much more sense logically rather than assigning functions which can not be observed onto these dead particles which can only be seen after heavy sample altering processes such as fixation, dehydrating, staining, and embedding which are used for electron microscopy preparation.
It is important to note that exosomes, like “viruses,” are regularly “isolated” through the process of cell culture. Many of us who challenge the evidence for the existence of “viruses” state that the particles seen in EM are most likely nothing more than cellular debris created through the culturing process. While the cell is kept outside the body in unnatural conditions, it is bombarded with antibiotics, antifungals, foreign DNA/materials, minimal nutrients, and physiologically unsuitable conditions. After being incubated for days, the cell is usually blasted with fresh heapings of many of the previously listed components and incubated further until the cell begins to break apart. While the cellular breakdown observed has been coined the cytopathogenic effect, it is a part of the process of cell death that is blamed on the invisible “virus.” And it is a fact that this very process of cell culturing can lead to the process of cell death known as apoptosis:
“Apoptosis is a genetically regulated process by which cells can be eliminated in vivo in response to a wide range of physiological and toxicological signals. Cells in vitro may be induced to die by apoptosis, e.g., by depletion of nutrients or survival factors from the culture media.”
Thus, it should be easy to see that these particles which have been called exosomes, apoptotic bodies, extracellular vesicles, “viruses,” etc. are created from the very cell destroying processes that the cell is put through in order to find the particles later in EM imaging. They are not the cause of the cell death but are the effect; a creation resulting from the process. Once the sample is put through purification steps such as ultracentrifugation and ultrafiltration, the bigger cellular debris particles are broken apart and eventually separated into smaller particles through unnaturally high g-forces and various chemical means. These particles are further altered during preparation for EM imaging and are presented as many different entities with varying theoretical functions applied to the same dead waste products.
The Exosome Concept
We already know that “viruses” began first as an idea in the early 1900’s once it was discovered that bacteria were unable to be blamed for every disease and were also found regularly in healthy subjects. It was assumed that there must be something smaller than bacteria in the fluids causing disease. The concept of the “virus” came before there was ever any evidence submitted for the existence of this invisible entity. Over 100 years later, we still have no direct evidence as to the existence of “viruses,” only indirect evidence used to infer their existence. And so it goes with exosomes which also started off as a concept before the entities were ever indirectly inferred into existence:
“The concept of exosomes was first proposed by Trams et al (1) in 1981, while soon after, exosomes were identified in a study of reticulocyte differentiation as a consequence of multivesicular endosome fusion with the plasma membrane.”
https://www.spandidos-publications.com/10.3892/ijmm.2018.3944#b2-ijmm-43-01-0083
As I was intrigued by how the idea of exosomes came about, I decided to break down the 1981 Trams paper in order to see what I could find out. What you will see, upon reading this study, is that just like their “viral” counterparts, the particles claimed to be exosomes were first visually recognized in cell culture fluids. In this study, many cell lines were used to look for the particles eventually picked as the representation for exosomes. They included:
- Established cultures
- Mouse neuroblastomas, N-18 and NB41A3
- Rat glioma, C-6
- Mouse melanoma, B-16
- Derived from embryonic or neonatal tissue as primary cultures
- Rat aorta, RA-B
- Mouse astroblast, D-34
- Grown from biopsy material
- Human melanoma, CL
- Human foreskin fibroblasts, KIN
The researchers noticed that in their studies on two enzymes, ecto-ATPases and ecto-5′-nucleotidases, these enzymes were released into the superfusate media of cultured cell lines. Due to their measuring of these two enzymes in the cultured cell media, the researchers decided to go looking for a cause. They proceeded to passage many cell lines and regularly tested the enzyme levels. The researchers eventually filtered the superfusate and subjected it to electron microscopy. After fixation of the pellets in buffered glutaraldehyde, they discovered two populations of vesicles; one which consisted of irregularly shaped vesicles approximately 500 to 1000 nm in diameter and another within the larger vesicles which was a population of smaller, spherical vesicles with an average size of about 40 nm. They then determined that these particles were the cause of their enzymatic effect without ever directly proving this by utilizing the scientific method.
Interestingly, upon finding these various particles, the researchers admitted that the vesicles could be fragments from the dying of lysed cells. Lysis is the breaking down of the membrane of a cell which is said to be caused by “viral,” enzymic, or osmotic mechanisms. In other words, these particles claimed as exosomes were possibly caused by the same process which creates “viral” particles when the cell breaks down as well as that which releases apoptotic bodies as the cell dies from apoptosis. This means that exosomes, “viruses,” apoptotic bodies, etc. are all the same particles released as the cell dies after being subjected to toxic conditions, such as the culturing of the cells for experimentation. They were just given different names and functions by different researchers.
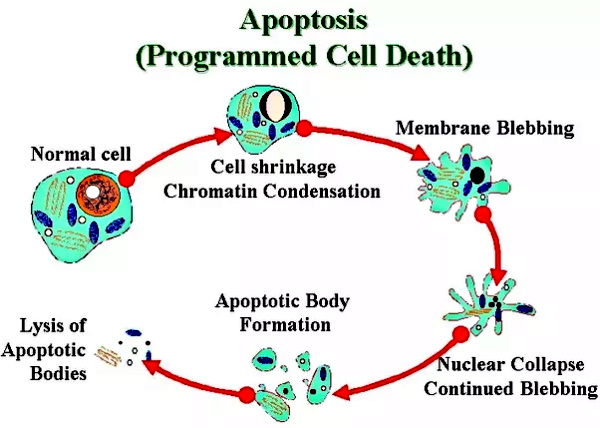
Trams et. al attempted to state, through indirect compositional differences based off of enzymatic readings of unpurified preparations, that these particles were not the product of lysed cells. However, they admitted that their smaller particles resembled vesicles “purified” from pig brain or from calf, rat and rabbit brain, while some of the more densely shadowed small vesicles resembled C-type “virus” particles. In other words, exosomes resembled “viruses” (which come from lysed cells) and the same exact particles were being found everywhere, not just in virology studies. These particles were being found in entirely healthy cell lines and in cultures containing no “viral” material whatsoever. Oddly enough, upon trying to find these same particles in the blood, they concluded that there was no firm evidence that plasma membrane derived microvesicles were present in the circulation. As the results came only from the cell culture process, the researchers wondered if the shedding of microvesicles and their interaction with a target cell or target organ represents a physiologic phenomenon that takes place in vivo (i.e. within a living organism)?
Obviously, this revelation of finding “virus” particles in healthy cultures would destroy the cell culture technique as being valid for “viruses” (even though John Franklin Enders admitted to finding measles “virus” particles in cultures without measles material). This type of study actually shows that “virus-like” particles are found within cell cultures without “viral” material, thus serving as a control of sorts for virology, the likes of which it regularly ignores. This obviously could not stand so these particles had to be something new. While no proof for the functioning of these particles was provided, a hypothesis was established. The researchers concluded that the intercellular transport of some trophic substances or nutrients might involve such vehicles as the microvesicles which they harvested from cell culture superfusates. As this could be a possibility, they decided to refer to these particles as exosomes rather than “viruses.” Thus the exosome concept was born.
The full 1981 Trams paper is presented below:
Exfoliation of membrane ecto-enzymes in the form of micro-vesicles
“Cultures from various normal and neoplastic cell lines exfoliated vesicles with 5′-nucleotidase activity which reflected the ecto-enzyme activity of the parent monolayer culture. The ratio of 5′-nucleotidase to ATPase activity in the microvesicles indicated that cellular ecto-ATPase was conserved in the exfoliative process. Phospholipids of the microvesicles contained significantly increased amounts of sphingomyelin and total polyunsaturated fatty acids. It was concluded that the shedded vesicles constituted a select portion of the plasma membrane. Examination by electron microscopy showed the vesicles had an average diameter of 500 to 1000 nm and often contained a second population of vesicles about 40 nm in diameter. As much as 70% of the plasma membrane ecto-5′-nueleotidase activity of a culture was released into the medium over a 24-h period. Phosphoesterhydrolases from C-6 glioma or N-18 neuroblastoma microvesicles dephosphorylated cell surface constituents when in contact with monolayer cultures. Exfoliated membrane vesicles may serve a physiologic function; it is proposed that they be referred to as exosomes.
Introduction
Plasma membrane ecto-ATPases and ecto-5′-nucleotidases have been found and characterized in a variety of eukaryotic cells and it is probable that each enzyme subserves more than one function on the cell surface. Both enzymes exhibit a broad specificity for the base moiety of nucleotide substrates [1] but it is not established that ATP or AMP are the predominant endogenous substrates. Ecto-ATPases have the properties of glycolipoproteins and are rather firmly bound to the plasma membrane, while ecto-5′-nucleotidases are composed of glycoprotein which appears to be collocated with sphingomyelin in situ and can be removed from the membrane matrix by fairly mild procedures [2]. During our investigations on the functional roles of these two ecto-enzymes we have observed that ATPase (EC 3.6.1.3) and 5′-nucleotidase (EC 3.1.3.5) were released into the superfusate media of cultured cell lines. We established that this release was not caused by cytolysis of moribund cells. The enzymes were released in the form of vesicles which are probably derived from specific domains of the plasma membrane. Whether or not the exfoliated microvesicles mediate physiologic processes in vivo has not been established.
Methods and Materials
Cell cultures. Cell lines employed in this study were established cultures (e.g. mouse neuroblastomas, N-18 and NB41A3; rat glioma, C-6; mouse melanoma, B-16), or derived from embryonic or neonatal tissue as primary cultures (rat aorta, RA-B; mouse astroblast, D-34) or grown from biopsy material (human melanoma, CL; human foreskin fibroblasts, KIN). Cells were grown in the appropriate medium as monolayers in 75 cm 2 plastic flasks (Falcon Plastics, Oxnard, CA) or on 530 cm 2 NUNC Bioassay dishes (A/S NUNC, Roskilde, Denmark). Passage numbers for a culture refer to the number of times the stock cell line has been subcultured by trypsinization, dilution and explantation into maintenance or experimental culture vessels. In particular, we have used the term ‘low passage’ for the rat glioma cell line C-6 when the parent cell was obtained from the American Type Culture Collection (Rockville, MD) at the earliest available passage (P-38). During repeated passage of this line we have observed over a number of years that ecto-5′-nucleotidase activity decreased sharply after about 20 passages and that ecto-ATPase activity increased. The term low passage is used for the C-6 line for P-38 to P-55 and high passage for passages P-65 to P-160.
Enzyme assays. ATPase activity was assayed on intact monolayer cultures or on isolated vesicles by a modified method of Weil-Malherbe and Green [3] by addition of [r 32p] ATP (New England Nuclear Corp., Boston, MA) to a superfusate buffer or to the vesicle suspension. The activity of 5′-nucleotidase was determined in a similar manner with [32p]AMP as substrate (New England Nuclear Corp.). Complete tissue culture growth media usually contain traces of ATPase and 5′-nucleotidase derived from the fetal calf serum component. Therefore, the cultures were washed prior to each experiment several times with a modified medium devoid of serum and routine incubations were performed in serum free media. We have used the term superfusate for modified media which were applied to confluent monolayer cultures in which enzyme accumulation was measured.
Lipid analyses. Phospholipid distribution in intact cells or extruded vesicles was estimated by two-dimensional TLC of a chloroform-methanol extract (2:1, v/v) according to Rouser et al. [4]. After development of the chromatogram, the TLC plates were charred with 50% (NH4)HSO4 and phosphate content of individual spots was determined by the method of Nelson [5]. For fatty acid analysis, aliquots of total lipid extracts were evaporated to dryness and methylated with BFa in methanol according to Morrison and Smith [6]. The fatty acid methyl esters were resolved and quantified on a Hewlett Packard 5840 gas chrom7atograph employing an SP 2330 column operated at 190°C.
Results
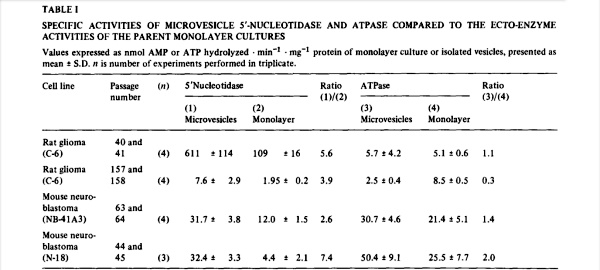
We have found that 5′.nucleofidase and ATPase were released into serum-free medium (superfusates) of monolayer cultures of normal and neoplastic cells. When a comparison was made between the ratio of ecto-5′-nucleotidase to ecto-ATPase activity in several cell lines and the activity of the two enzymes released into medium over a 24-h period, it was found that there was a proportionately larger release of 5′-nucleotidase (Table I). As we shall demonstrate below, the released enzymes had been derived from the corresponding plasma membrane ecto-enzymes. The relative preponderance of 5′-nucleotidase over ATPase in the microvesicles, compare ratios (1)/(2) to (3)/(4), indicated that either the ATPases were more labile, or that they had been conserved. When the decay of the catalytic activity of the released enzymes was measured by continued incubation in cell-free medium, it was found that 5′-nucleotidase lost from 3 to 20% of its activity in 24 h while the released ATPase averaged a catalytic loss of about 33% in the same period. Therefore, while the ATPases were somewhat more labile than the 5′-nucleotidases, the 2- to 13-fold enrichment of 5′-nucleotidase in the released microvesicles suggested a conservation of plasma membrane
ecto-ATPases.
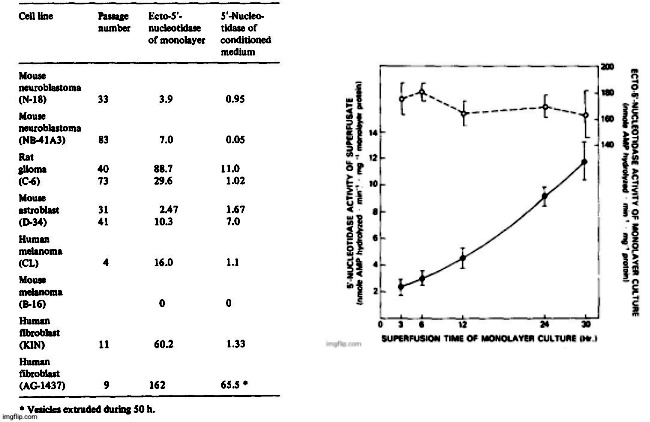
The release of 5′-nucleotidase activity into 24-h superfusates ranged from 2 to 70% of measured monolayer ecto-5′-nucleotidase activity and it was characteristic for a particular cell line and passage number. With increasing passage number, ecto-5′-nucleotidase/ecto-ATPase activity ratios changed in several cell lines and the amount of enzymes released into superfusates also changed. While duplication was satisfactory when measurements were made within a few days or within a few passages, comparisons made several months apart were not amenable to statistical treatment.
The results diplayed in Table II on the release of 5′-nucleotidase from a variety of cell lines should be viewed as representative. Release of the enzyme was found to be low from the NB-41A3 mouse neuroblastoma clone and highest in a primary culture derived from neonatal mouse astroblasts (D-34). Only in superfusates from mouse melanoma B-16 was there no measurable enzyme activity released into superfusates, but there was also no detectable ecto-5′-nucleotidase in the monolayer cultures. The rate of enzyme accumulation in the superfusates was linear with time in low density cultures but increased somewhat when cell density was high as shown for two separate duplicate experiments on the rat glioma cell line (Fig. 1). The rate of ATPase accumulation (not shown in Fig. 1) was very similar to that obtained with 5′-nucleotidase. The C-6 glioma culture generally exhibits a high ecto-5′-nucleotidase activity at low passage but the specific activity of the ecto-enzyme does not change substantially over a 30-h period (Fig. 1).
The rate of enzyme liberation was not changed significantly by modification of fetal calf serum concentration in the medium (0 to 20%) or by the addition of 0.5% trypsin to the medium. The release of 5′-nucleotidase activity into superfusates was altered by several compounds; in C-6 glioma cultures the extrusion of enzyme was inhibited by 93 +_ 3% in the presence of 10-6M concanavalin A. With 10 -s M cycloheximide, inhibition was 32 + 24% over a 24-h period. An increase of enzyme extrusion was found in the presence of 10 -6 M colchicine (141 + 35% over control) or when the medium contained 0.5 ug. m1-1 of cytochalasin B (95 -+ 43% over control).
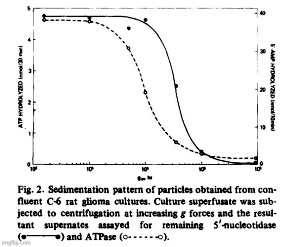
Filtration of superfusates showed that from 97 to 99% of 5′-nucleotidase activity was retained on 0.22 um filters while about 80% passed through an 0.45 um filter. The released enzyme activity was particulate and the particles could also be harvested by centrifugation. In Fig. 2, we show residual medium ATPase and 5′-nucleotidase after subjecting superfusate from glioma cultures (C-6) to increasing centrifugal forces. Cellular debris and unattached cells sedimented at or below 5 • 10^3 • gh (Sorvall SS-34 rotor at 10 a Xg for 0.5 h). The particulate enzymes contained in those supernates could be collected by centrifugation at high speeds. For routine collections of extruded enzyme, the Sorvall supernates were centrifuged for 90 min in a Spinco Ti-70 rotor at 310 000 × g. The small gelatinous pellet could be removed in toto or resuspended in buffer. ATPase activity sedimented at a faster rate than 5′-nucleotidase which indicated that the particle population was not homogeneous. Electronmicroscopy after fixation of the pellets in buffered glutaraldehyde revealed two populations of vesicles, one of which consisted of irregularly shaped vesicles approximately 500 to 1 000 nm in diameter. Contained within those vesicles was another population of smaller, spherical vesicles with an average size of about 40 nm (Fig. 3).
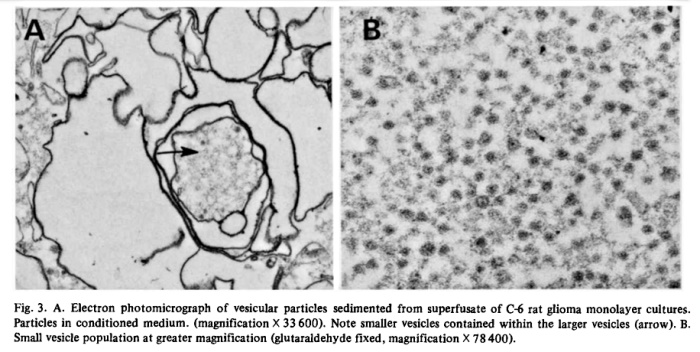
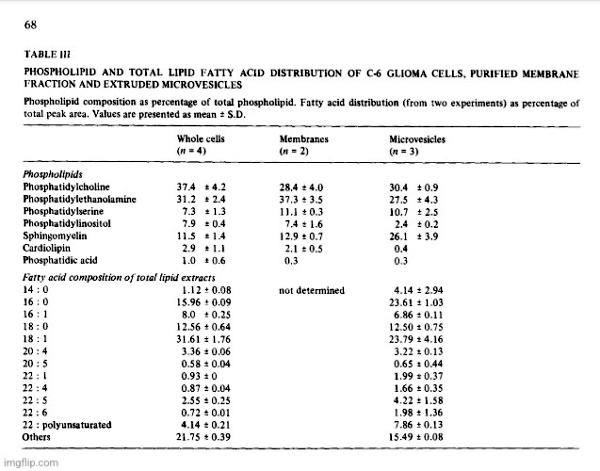
Conceivably, the vesicles were fragments from dying of lysed cells, but the liberation of as much as 70% of its 5′-nucleotidase activity from a healthy monolayer culture in 24 h would result in the accumulation of many other subcellular fragments if that were the case. Analysis of a representative high speed pellet of 6.5 mg protein from rat glioma superfusates yielded 5′-nucleotidase activity of 1.003 panol AMP hydrolyzed • min -1 • mg -1 protein, while marker enzymes for other subcellular particles were virtually absent. Activities of glucose-6-phosphatase (EC 3.1.3.9), cytochrome c oxidase (EC 1.9.3.1) and N-acetylhexosaminiclase (EC 3.2.1.52) were nil and (Na ÷, K+)-ATPase (EC 3.6.1.3) was low (25 nmol • min -1 • mg -1 protein). The 5′-nucleotidase/LDH ratio in C-6 conditioned medium was several fold higher than in cell homogenates and there was no DNA detectable in sedimented vesicles. A comparison of the optimal requirements for divalent cations of the released ATPase showed that stimulating and inhibitory concentrations of Mg 2+, Ca 2+ and Mn 2+ were identical with those required for the respective monolayer ecto-ATPase. Ecto-5′-nucleotidases have a high binding affinity for concanavalin A and about 70% of the nucleotidase activity of C-6 conditioned media was retained by a Sepharose-4G-Con A column, suggesting also a similarity between the ecto-enzyme and the released enzyme. Analysis of vesicle pellets from glioma superfusates disclosed an RNA content of about 5% and lipid content of 30 to 40%. Two-dimensional TLC of vesicle phospholipids [4] gave a pattern which was different from that of lipid extracts of whole cells and from plasma membrane preparations in which 5′-nucleotidase was enriched about 8-fold (Table III). The vesicles contained significantly increased amounts of sphingomyelin and decreased phosphatidylinositol. Comparison of total lipid fatty acid composition of whole cells with vesicles showed that the latter contained increased palmitic acid and total polyunsaturated fatty acids and decreased oleic acid. These compositional differences were further evidence that the exfoliated vesicles had not been derived from lysed cells.
That the vesicles had been derived from the plasma membrane of the respective monolayer cell lines was suggested by the observation that the specific activities of microvesicle and monolayer enzymes were roughly of the same order of magnitude (Table I). Both 5′-nucleotidase and ATPase are classical plasma membrane marker enzymes, but the conservation of ATPase in the exfoliative process strongly suggests that the microvesicles were derived from specific domains of the plasma membrane. Another plasma membrane marker GM 1 (as measured by cholera toxin binding) was not conserved (Salem, N., Lauter, C.J. and Trams, E.G., unpublished results). This may indicate, that ecto-5′-nucleotidase and ecto-ATPase do not serve an interdependent function on the cell surface, as for instance in the catabolism of translocated cytoplasmic ATP [2].
The morphologic similarity of the extruded vesicles to synaptosomal preparations suggested a possible transport function for them. Cells transfer substances to target cells in order to support discrete functions and examples of trophic substances are fibroblast- or nerve growth-factors [7,8].
Our working hypothesis was that one or more of the ecto-phosphoester hydrolases might play a role in a recognition and/or transport process. For instance, the carbohydrate moiety of ecto-5′-nucleotidase might serve as an address which was recognized by a recipient cell and the catalytic moiety of the enzyme would serve to dephosphorylate a receptor constituent and thereby facilitate a transfer mechanism between vesicle and cell. To test this hypothesis, mouse neuroblastoma cells (N-18) were incubated with 32Pi-containing medium with the intent to label cell surface phosphorous-containing compounds. After removal of the isotopic incubation medium, the N-18 cultures were first washed with unlabeled medium and then vesicle suspensions harvested from C-6 glioma conditioned medium were added; normal culture medium served as a control. There was a significant increase in 32p release into the medium (over background 32p diffusion from the cells) when gila-derived vesicles were in contact with the neuroblastoma monolayer cultures (Table IV). In another experiment, 32P-prelabeled C-6 cultures were superfused with either C-6 or with N-18 vesicles. There was a larger release of 32p when glioma cells were incubated with N-18 derived vesicles than when they were incubated with homologous vesicles which suggested that there were either quantitative or qualitative differences between the two experiments. We have no evidence at present to show that the increases of 32p release in the presence of the vesicles was due only to dephosphorylation of cell surface constituents, but the experiments indicate that some interaction between the monolayer cells and the vesicles had taken place.
Because the release of microvesicles occurred in all cell-lines which we have studied so far, we conducted some preliminary tests for their presence in the circulation. Plasma levels of 5′-nucleotidase may be elevated significantly in several diseases [9,10] and the enzyme might normally or pathologically be derived
from plasma membranes. We assumed that the presence of such vesicles would be recognizable by their enzyme activity after filtration or centrifugation of blood plasma. We assayed heparinized blood from 16 randomly selected patients and found plasma 5′-nucleotidase activities ranging from 3.4 to 26 nmol AMP hydrolyzed • min -1 • m1-1 plasma. Only a minor fraction of that activity was sedimentable, however, or retained on Millipore filters and there is at present no firm evidence that plasma membrane derived microvesicles are present in the circulation.
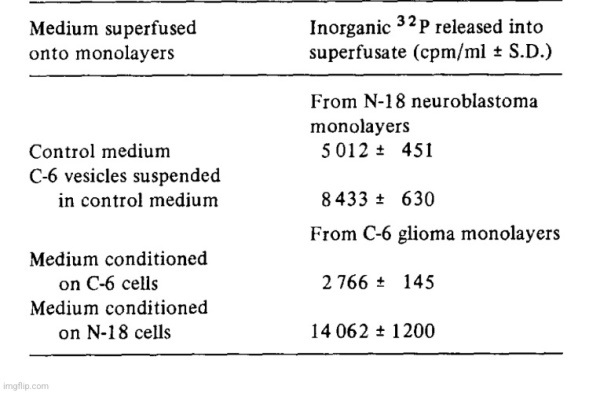
Discussion
Our observations suggest that exfoliation of membranous vesicles might occur in many different normal and neoplastic cells. The accumulation of as much as 70% of plasma membrane 5′-nucleotidase in microvesicular form in the medium over a 24-h period suggests a fairly high membrane tumover. This is not
extraordinary, because it has been calculated that macrophages and L-cells were capable of interiorizing the equivalent of their cell surface every 33 and 125 min, respectively [11]. Replacement of apical plasma membrane in the lactating mammary gland requires formidable capapcity for membrane synthesis [12] and replacement of exfoliated membrane is a requirement that presumably is easily met by most cells. We have presented evidence that the microvesicles harvested from tissue culture superfusates were not mere fragments from the cytolysis of moribund cells. The preferential release of plasma membrane ecto-5′-nucleotidase over ecto-ATPase furthermore suggests that the exfoliative process was selective and that the microvesicles consisted of specific domains of the plasma membrane. The substantial enrichment of sphingomyelin in the microvesicular fraction supports this contention. A similar fmding of increased sphingomyelin in extracellular membranous vesicles associated with a murine ascitic leukemia was reported by Van Blitterswijk et al. [13]. Microvillous membrane accumulation in media of cultured chick embryo intestines was observed recently by Black et al. [14] and extracellular membrane-invested vesicles have been described by Anderson [15]. The latter particles appear to play a role in mineralization processes and they have been referred to as matrix vesicles. Their size ranged from 300 to 1000 nm and it was postulated that they were derived from the plasma membrane of chondrocytes by budding [15]. Their lipid composition was very similar to that of chondrocyte plasma membrane [16] and similar to the lipid composition of the vesicles which we have collected from rat glioma cultures. The electronmicroscopic images of the particles from our rat glioma culture superfusates suggest that the larger membranes were of plasmalemma origin. The smaller population has some similarities to vesicles purified from pig brain [17] or from calf, rat and rabbit brain [18], while some of the more densely shadowed small vesicles resemble C-type virus particles (Todaro, G., personal communication).
The dephosphorylation, presumably of monolayer cell surface components by microvesicle ecto-phosphoesterhydrolases, suggested an interaction between vesicles and cells. We also have recently found that isotopically labeled constituents of the microvesicles can be transfered to recipient cells (Trams, E.G., Lauter, C.J. and Salem, N., unpublished results) and the question must be asked if the shedding of microvesicles and their interaction with a target cell or target organ represents a physiologic phenomenon that takes place in vivo? Inter-cellular transfer of a quantum of material by means of vesicles has been recognized in neurochemical transmission and there is evidence that metabolic cooperation by packaged transfer of substances may occur elsewhere, such as the transport of macromolecules between glia and neurons [19-21]. It is also conceivable that the vesicle in part or in toto can be incorporated into a recipient cell, thereby producing a modification of the host cell. Such an effect was observed when exfoliated vesicles from a B-16 mouse melanoma subline were fused experimentally with cells from another B-16 subline [22]. Attempts are made currently in several laboratories to design packaged substances for targeted therapeutic use. As an example, liposomes are provided with an organ-specific address [23] and it is hoped that such models will find application, for instance in the treatment of metabolic dystrophies by enzyme replacement. Conceivably, the physiologic distribution of some cellular products between cells or organs is achieved in a similar way, i.e. they are packaged and provided with an address, rather than simply diffused through extracellular fluid compartments. The inter-cellular transport of some trophic substances or nutrients might involve such vehicles as the microvesicles which have been harvested from cell culture superfusates. In a preliminary report we have suggested that such plasma membrane derived vesicles could be referred to generically as exosomes [24].”
doi: 10.1016/0005-2736(81)90512-5.
In Summary:
- Exosomes and “viruses” can not be separated from each other (as they are the same particles) which has created a problem for researchers:
1. How can exosome researchers be sure that they are isolating and quantifying extracellular vesicles rather than enveloped “viruses” present in the sample?
2. How can “viral” researchers know that they are not detecting similarly sized “non-viral” vesicles or empty vectors?
- It is currently virtually impossible to specifically separate and identify EVs that carry “viral” proteins, host proteins, and “viral” genomic elements from enveloped “viral” particles that carry the same molecules
- To date, a reliable method that can actually guarantee a complete separation of these particles does not exist
- Exosomes have been disregarded as cellular debris and as garbage carriers and were once thought to be biomarkers of a diseased state
- They are now thought to be biologically active
- Despite 20 years of research, the very basics of exosome biology are in their infancy and we know little of the part they play in normal cellular physiology (i.e. it is all guesswork)
- Other particles said to be garbage bags as well as carriers of cellular information are apoptotic bodies created during apoptosis, a process of cell death:
- Cell shrinks
- Cell fragments
- Cytoskeleton collapses
- Nuclear envelope disassembles
- Cells release apoptotic bodies
- Apoptotic bodies, ectosomes and exosomes are all roughly the same size (typically 40–100 nm) and all also contain cytosol
- Understanding differences between them is of paramount importance but has too often been overlooked
- Cells in vitro (i.e. cell culture) may be induced to die by apoptosis, e.g., by depletion of nutrients or survival factors from the culture media
- The exosome concept was created by Trams et. al in 1981
- Exosomes were first “discovered” in cell cultures and were admitted to potentially be cellular debris
- In other words, exosomes=”viruses”=apoptotic bodies=cellular debris

- Cultures from various normal and neoplastic cell lines exfoliated vesicles with 5′-nucleotidase activity which reflected the ecto-enzyme activity of the parent monolayer culture
- Examination by electron microscopy showed the vesicles had an average diameter of 500 to 1000 nm and often contained a second population of vesicles about 40 nm in diameter
- Exfoliated membrane vesicles may serve a physiologic function; it is proposed that they be referred to as exosomes
- In other words, the particles came from cell cultures and ranged anywhere from 40 to 1000 nm, showing that these were not purified preparations of a single substance
- During the investigations on the functional roles of two ecto-enzymes, the researchers stated that they “observed” that ATPase and 5′-nucleotidase were released into the superfusate media of cultured cell lines
- They claimed to have established that this release was not caused by cytolysis (the dissolution or disruption of cells, especially by an external agent) of moribund cells
- The enzymes were released in the form of vesicles which were probably derived from specific domains of the plasma membrane
- Whether or not the exfoliated microvesicles mediate physiologic processes in vivo (in the living body) had not been established
- In other words, they found particles in the size range of “viruses” which they decided were not a product of cell disintegration by pathological means and assumed they were different and provided functions without direct proof
- Cell lines employed in this study were:
- Established cultures
- Mouse neuroblastomas, N-18 and NB41A3
- Rat glioma, C-6
- Mouse melanoma, B-16
- Derived from embryonic or neonatal tissue as primary cultures
- Rat aorta, RA-B
- Mouse astroblast, D-34
- Grown from biopsy material
- Human melanoma, CL
- Human foreskin fibroblasts, KIN
- Established cultures
- Cells were grown in the appropriate medium as monolayers in 75 cm 2 plastic flasks
- Passage numbers for a culture refer to the number of times the stock cell line has been subcultured by trypsinization, dilution and explantation into maintenance or experimental culture vessels
- During repeated passage of the rat glioma cell line C-6, they observed over a number of years that ecto-5′-nucleotidase activity decreased sharply after about 20 passages and that ecto-ATPase activity increased
- Complete tissue culture growth media usually contain traces of ATPase and 5′-nucleotidase derived from the fetal calf serum component
- Therefore, the cultures were washed prior to each experiment several times with a modified medium devoid of serum and routine incubations were performed in serum free media
- They used the term superfusate for modified media which were applied to confluent monolayer cultures in which enzyme accumulation was measured
- They found that 5′.nucleofidase and ATPase were released into serum-free medium (superfusates) of monolayer cultures of normal and neoplastic cells
- The release of 5′-nucleotidase activity into 24-h superfusates ranged from 2 to 70% of measured monolayer ecto-5′-nucleotidase activity and it was characteristic for a particular cell line and passage number
- With increasing passage number, ecto-5′-nucleotidase/ecto-ATPase activity ratios changed in several cell lines and the amount of enzymes released into superfusates also changed
- While duplication was satisfactory when measurements were made within a few days or within a few passages, comparisons made several months apart were not amenable to statistical treatment
- In other words, the results related directly to the cell line used and the amount of passages performed and duplication was not satisfactory after a few months
- The rate of enzyme liberation was not changed significantly (i.e. there was a change) by modification of fetal calf serum concentration in the medium (0 to 20%) or by the addition of 0.5% trypsin to the medium
- The release of 5′-nucleotidase activity into superfusates was altered by several compounds
- Thus we can see that adding compounds can alter the results obtained
- ATPase activity sedimented at a faster rate than 5′-nucleotidase which indicated that the particle population was not homogeneous (i.e. it was a mixed population of different particles)
- Electronmicroscopy after fixation of the pellets in buffered glutaraldehyde revealed two populations of vesicles:
- One of which consisted of irregularly shaped vesicles approximately 500 to 1000 nm in diameter
- Contained within those vesicles was another population of smaller, spherical vesicles with an average size of about 40 nm
- FYI: exosomes are said to be anywhere from 30-150 nm meaning this was not strictly the presumed exosomes in the mixture, i.e. not purification/isolation
- Conceivably, the vesicles were fragments from dying of lysed cells, but they excuse this conclusion due to the liberation of as much as 70% of its 5′-nucleotidase activity from a healthy monolayer culture in 24 h as they claim this would result in the accumulation of many other subcellular fragments if that were the case
- They looked to compositional differences to provide further evidence that the exfoliated vesicles had not been derived from lysed cells (yet, without purifying and isolating the particles, how would compositional differences be ascertained…?)
- That the vesicles had been derived from the plasma membrane of the respective monolayer cell lines was suggested by the observation that the specific activities of microvesicle and monolayer enzymes were roughly of the same order of magnitude
- They claim both 5′-nucleotidase and ATPase are said to be classical plasma membrane marker enzymes, but the conservation of ATPase in the exfoliative process strongly suggested that the microvesicles were derived from specific domains of the plasma membrane
- The morphologic similarity of the extruded vesicles to synaptosomal preparations suggested a possible transport function for them (i.e. the particles looked the same as those found in cultures from the brain)
- The working hypothesis was that one or more of the ecto-phosphoester hydrolases might play a role in a recognition and/or transport process
- They carried out two experiments to test this hypothesis and concluded that they had no evidence at present to show that the increases of 32p release in the presence of the vesicles was due only to dephosphorylation of cell surface constituents, but they felt the experiments indicated that some interaction between the monolayer cells and the vesicles had taken place
- Because the release of microvesicles occurred in all cell-lines which were studied, they conducted some preliminary tests for their presence in the circulation
- They assumed that the presence of such vesicles would be recognizable by their enzyme activity after filtration or centrifugation of blood plasma
- After testing, they concluded that there was no firm evidence that plasma membrane derived microvesicles are present in the circulation
- The researchers felt that their observations suggest that exfoliation of membranous vesicles might occur in many different normal and neoplastic cells
- They claimed to have presented evidence that the microvesicles harvested from tissue culture superfusates were not mere fragments from the cytolysis of moribund cells (which they admitted to be a conceivable possibility)
- The preferential release of plasma membrane ecto-5′-nucleotidase over ecto-ATPase furthermore suggested that the exfoliative process was selective and that the microvesicles consisted of specific domains of the plasma membrane
- The electronmicroscopic images of the particles from their rat glioma culture superfusates suggested that the larger membranes were of plasmalemma origin
- The smaller population had some similarities to vesicles purified from pig brain or from calf, rat and rabbit brain, while some of the more densely shadowed small vesicles resemble C-type “virus” particles
- In other words, they found the exact same particles seen in animal brain cultures as well as “viruses” but assigned them a different name and function based on indirect chemical results from mixed unpurified preparations coming from cell cultures
- The dephosphorylation, presumably of monolayer cell surface components by microvesicle ecto-phosphoesterhydrolases, suggested an interaction between vesicles and cells
- They stated that the question must be asked if the shedding of microvesicles and their interaction with a target cell or target organ represents a physiologic phenomenon that takes place in vivo?
- In other words, they did not know whether the process they created in their culture soup actually occurs within a living organism
- It is also conceivable (i.e. capable of being imagined) that the vesicle in part or in toto can be incorporated into a recipient cell, thereby producing a modification of the host cell (sounds like a “virus…”)
- Conceivably, the physiologic distribution of some cellular products between cells or organs is achieved in a similar way, i.e. they are packaged and provided with an address, rather than simply diffused through extracellular fluid compartments
- The inter-cellular transport of some trophic substances or nutrients might involve such vehicles as the microvesicles which have been harvested from cell culture superfusates
- In a preliminary report they suggested that such plasma membrane derived vesicles could be referred to generically as exosomes
“Since vesicles resemble viruses, the question of course is whether the first extracellular vesicles were primitive viruses and the viruses learned from extracellular vesicles or vice versa.”
“Viruses can replicate and vesicles cannot. But there are many variants in between. Where do viruses start, and where do extracellular vesicles start?”
~ Leonid Margolis
https://www.quantamagazine.org/cells-talk-in-a-language-that-looks-like-viruses-20180502/
We need to be careful replacing one fraudulent theory with another. Sadly, many have fallen into this trap of scraping the “virus” concept and replacing it with the exosome concept. What they do not realize is that these two concepts are built upon the same fraudulent foundation. Both are tied to the cell culture process and come from the same cell death initiated by toxilogical overload. This is why researchers are having a hard time separating not only the particles but also their theoretical functioning from each other. When the lies become overly complicated, they begin to entangle with each other and the illusion begins to fall apart.
Whatever name you want to call them, the broken down cellular debris known as exosomes, “viruses,” apoptotic bodies, extracellular vesicles, etc. are all the same particles consisting of the same size, density, and morphology. They are assigned different names and functions based on the researchers looking at them. While they are claimed to be separate entities, the particles are unable to be purified and isolated from everything else in order to be independently studied and characterized. Their functioning can not be observed within a living organism thus the same particles are given theoretical roles within the body based on the researchers performing the experiments. None of these particles have met the burden of proof of being established through rigorous testing and adherence to the scientific method. As they can never be observed in nature and must be created to be “seen,” they fail the very first criteria. As they can not be separated, they fail at being a valid independent variable. Without a valid independent variable, cause and effect can not be determined. This means that the scientific method can not and is not being applied to these particles. Thus all of the indirect evidence accumulated for this cellular debris assuming multiple identities is nothing but pseudoscientific fairy tales.
cover image credit: geralt

Truth Comes to Light highlights writers and video creators who ask the difficult questions while sharing their unique insights and visions.
Everything posted on this site is done in the spirit of conversation. Please do your own research and trust yourself when reading and giving consideration to anything that appears here or anywhere else.











