COVID Vaccine Injury Reports Jump by 27,000 in One Week, FDA Pulls ‘Bait and Switch’ With Pfizer Vaccine Approval
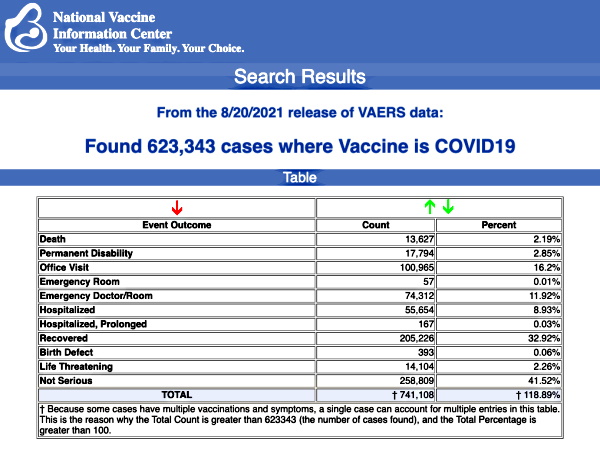
VAERS data released Friday by the CDC showed a total of 623,343 reports of adverse events from all age groups following COVID vaccines, including 13,627 deaths and 84,466 serious injuries between Dec. 14, 2020 and Aug. 20, 2021.
by Megan Redshaw, The Defender
August 27, 2021
Data released today by the Centers for Disease Control and Prevention (CDC) showed that between Dec. 14, 2020 and Aug. 20, 2021, a total of 623,343 total adverse events were reported to VAERS, including 13,627 deaths — an increase of 559 over the data released last week.
There were 84,466 reports of serious injuries, including deaths, during the same time period — up 3,416 compared with the previous week.
Excluding “foreign reports” filed in VAERS, 488,318 adverse events, including 6,128 deaths and 38,765 serious injuries, were reported in the U.S. between Dec. 14, 2020 and Aug. 20, 2021.between Dec. 14, 2020 and Aug. 20, 2021.
Of the 6,128 U.S. deaths reported as of Aug. 20, 13% occurred within 24 hours of vaccination, 18% occurred within 48 hours of vaccination and 32% occurred in people who experienced an onset of symptoms within 48 hours of being vaccinated.
In the U.S., 360.3 million COVID vaccine doses had been administered as of Aug. 20. This includes: 203 million doses of Pfizer, 143 million doses of Moderna and 14 million doses of the Johnson & Johnson (J&J).
The data come directly from reports submitted to the Vaccine Adverse Event Reporting System (VAERS), the primary government-funded system for reporting adverse vaccine reactions in the U.S.
Every Friday, VAERS makes public all vaccine injury reports received as of a specified date, usually about a week prior to the release date. Reports submitted to VAERS require further investigation before a causal relationship can be confirmed.
This week’s U.S. data for 12- to 17-year-olds show:
- 17,518 total adverse events, including 1,047 rated as serious and 18 reported deaths. Two of the 18 deaths were suicides.
The most recent reported deaths include a 15-year-old boy (VAERS I.D. 1498080) who previously had COVID, was diagnosed with cardiomyopathy in May 2021 and died four days after receiving his second dose of Pfizer’s vaccine on June 18, when he collapsed on the soccer field and went into ventricular tachycardia; and a 13-year-old girl (VAERS I.D. 1505250) who died after suffering a heart condition after receiving her first dose of Pfizer.
- Other deaths include two 13-year-old boys (VAERS I.D. 1406840 and 1431289) who died two days after receiving a Pfizer vaccine, a 13-year-old boy who died after receiving Moderna (VAERS I.D. 1463061), three 15-year-olds (VAERS I.D. 1187918, 1382906 and 1242573), five 16-year-olds (VAERS I.D. 1420630, 1466009, 1225942, 1475434, and 1386841) and three 17-year-olds (VAERS I.D. 1199455, 1388042 and 1420762).
- 2,609 reports of anaphylaxis among 12- to 17-year-olds with 99% of cases
attributed to Pfizer’s vaccine. - 444 reports of myocarditis and pericarditis (heart inflammation) with 438 cases attributed to Pfizer’s vaccine.
- 89 reports of blood clotting disorders, with all cases attributed to Pfizer.
This week’s total U.S. VAERS data, from Dec. 14, 2020 to Aug. 20, 2021, for all age groups combined, show:
- 21% of deaths were related to cardiac disorders.
- 54% of those who died were male, 43% were female and the remaining death reports did not include gender of the deceased.
- The average age of death was 73.1.
- As of Aug 20., 3,190 pregnant women reported adverse events related to COVID vaccines, including 982 reports of miscarriage or premature birth.
- Of the 2,640 cases of Bell’s Palsy reported, 50% were attributed to Pfizer vaccinations, 43% to Moderna and 7% to J&J.
- 530 reports of Guillain-Barré Syndrome, with 39% of cases attributed to Pfizer, 34% to Moderna and 26% to J&J.
- 132,694 reports of anaphylaxis with 43% of cases attributed to Pfizer’s vaccine, 49% to Moderna and 8% to J&J.
- 8,528 reports of blood clotting disorders. Of those, 3,633 reports were attributed to Pfizer, 3,101 reports to Moderna and 1,746 reports to J&J.
- 2,162 cases of myocarditis and pericarditis with 1,364 cases attributed to Pfizer, 714 cases to Moderna and 78 cases to J&J’s COVID vaccine.
BBC radio host died of COVID vaccine complications, coroner confirms
An award-winning BBC radio host died as a result of complications from her first dose of AstraZeneca’s COVID vaccine, coroner Karen Dilks concluded.
Lisa Shaw, 44, received her first dose of AstraZeneca on April 29. On May 13, she was taken by ambulance to University Hospital of North Durham after having a headache for several days. She was transferred to the Royal Victoria Infirmary in Newcastle, where she received a number of treatments, which included cutting away part of her skull to relieve the pressure on her brain. She died May 21.
According to the BBC, Tuomo Polvikoski, a pathologist, told the coroner Shaw was fit and healthy before receiving the vaccine. When asked about the underlying cause of the fatal clotting on her brain, Polvikoski said the clinical evidence “strongly supports the idea that it was, indeed, vaccine-induced.”
FDA grants full approval of Pfizer vaccine, critics blast agency for lack of data, scientific debate
The U.S. Food and Drug Administration (FDA) Aug. 23 granted full approval to Pfizer’s “Comirnaty” COVID vaccine for people 16 years and older — without allowing public discussion or holding a formal advisory committee meeting to discuss data.
This is the first COVID vaccine approved by the FDA, and is expected to open the door to more vaccine mandates by employers and universities.
According to The Washington Post, Pfizer’s vaccine approval was the fastest in the agency’s history, coming less than four months after Pfizer/BioNTech filed for licensing on May 7.
According to an article published Aug. 20 in the BMJ, transparency advocates criticized the FDA decision not to hold a formal advisory committee meeting to discuss Pfizer’s application for full approval — an important mechanism used to scrutinize data.
Last year the FDA said it was “committed to use an advisory committee composed of independent experts to ensure deliberations about authorisation or licensure are transparent for the public.”
But in a statement to The BMJ, the FDA said it did not believe a meeting was necessary ahead of the expected full FDA approval.
Kim Witczak, a drug safety advocate who serves as a consumer representative on the FDA’s Psychopharmacologic Drugs Advisory Committee, said it’s concerning that full approval is based on only six months’ worth of data — despite clinical trials designed for two years — and there’s no control group after Pfizer offered the product to placebo participants before the trials were completed.
FDA approval letter causes confusion, raises questions
Buried in the fine print of Monday’s approval of the Pfizer Comirnaty vaccine are two critical facts that affect whether the vaccine can be mandated, and whether Pfizer can be held liable for injuries, according to Children’s Health Defense Chairman Robert F. Kennedy, Jr. and Dr. Meryl Nass.
Here's my latest with @NassMeryl… Buried in fine print of Monday's approval by FDA of Pfizer Comirnaty COVID vaccine are 2 critical facts that affect whether vaccine can be mandated + whether Pfizer can be held liable for injuries.https://t.co/QtpHufCKDI
— Robert F. Kennedy Jr (@RobertKennedyJr) August 25, 2021
Kennedy and Nass, who accused the FDA of pulling a “bait and switch” on the public, said the FDA acknowledged that while Pfizer has “insufficient stocks” of the newly licensed Comirnaty vaccine available, there is “a significant amount” of the Pfizer-BioNTech COVID vaccine — produced under Emergency Use Authorization (EUA) — still available for use.
The FDA decreed that the Pfizer-BioNTech vaccine under the EUA should remain unlicensed — but that it can be used “interchangeably” (page 2, footnote 8) with the newly licensed Comirnaty product.
Second, the FDA said the licensed Pfizer Comirnaty vaccine and the existing EUA Pfizer vaccine are “legally distinct,” but said their differences do not “impact safety or effectiveness.”
Kennedy and Nass said EUA products are experimental under U.S. law. Both the Nuremberg Code and federal regulations provide that no one can force a human being to participate in this experiment.
Under 21 U.S. Code Sec.360bbb-3(e)(1)(A)(ii)(III), “authorization for medical products for use in emergencies,” it is unlawful to deny someone a job or an education because they refuse to be an experimental subject, they wrote.
At least for the moment, the Pfizer Comirnaty vaccine has no liability shield. Vials of the branded product, which say “Comirnaty” on the label, are subject to the same product liability laws as other U.S. products, Kennedy and Nass said, adding that “Pfizer is therefore unlikely to allow any American to take a Comirnaty vaccine until it can somehow arrange immunity for this product.”
On Thursday, Sen. Ron Johnson (R-Wis.) wrote the FDA raising similar concerns and questions about the agency’s approval of the Pfizer Comirnaty vaccine.
In his letter, Johnson asked FDA Acting Commissioner Dr. Janet Woodruff why the FDA didn’t grant full licensure for the Pfizer-BioNTech vaccine that is already in use and available in the U.S., and how the agency will ensure that those being vaccinated under mandates will receive the FDA-approved version.
As COVID surges among fully vaccinated, CDC fails to properly track breakthrough cases
As The Defender reported Aug. 24, the most recent data from the CDC shows 9,716 breakthrough cases resulting in hospitalization or death as of Aug. 16. However, the agency states those numbers are underreported.
On May 1, the CDC made a decision to stop tracking all breakthrough cases and instead only track cases in the fully vaccinated that resulted in hospitalization or death. That leaves public health officials without the full data that can answer questions as the new Delta variant spreads.
In an interview with PBS News Hour, Jessica Malaty Rivera, an infectious disease epidemiologist and research fellow at Boston Children’s Hospital and former science communications lead at the COVID Tracking Project, said not tracking breakthrough data with as much granularity as we would hope is “basically creating blind spots in our understanding of the true impact of the virus, especially the variants that are circulating so widely in the United States.”
The New York Times recently published data from seven states — California, Colorado, Massachusetts, Oregon, Utah, Vermont and Virginia — that keeps particularly detailed records on breakthrough cases.
Analysis showed that in six of the states, breakthrough infections made up 18% to 28% of all newly diagnosed cases of COVID in the past several weeks, and 12% to 24% of all COVID-related hospitalizations, with reported deaths higher than the CDC’s original estimate of .5%.
Pfizer scheme to churn out ‘variant-specific’ vaccines will lead to more variants, experts warn
Pfizer CEO Albert Bourla on Tuesday told Fox News the company has a system in place to turn around a variant-specific jab within 95 days in the likelihood a vaccine-resistant COVID strain emerges, but experts warn that strategy will backfire.
Bourla said Pfizer hasn’t identified any variants that could escape the vaccine yet. However, that statement contradicts the findings of numerous studies by the Centers for Disease Control and Prevention (CDC) which show waning immunity against the Delta variant.
Dr. Peter McCullough, board certified in internal medicine, cardiovascular diseases and clinical lipidology, said in a recent podcast: “There are clearly sources of information to suggest that once we start vaccination and we get more than 25% of the population vaccinated, we will allow one of the variants that’s in the background to emerge because it’s resistant to the vaccine.”
“That [theory] makes sense,” McCullough said. “Just like an antibiotic, once we get to a certain percentage of coverage with an antibiotic, we’ll allow a resistant bacteria to move forward.”
According to Dr. Robert Malone, inventor of mRNA and DNA vaccines, worldwide expert in RNA technologies and Harvard-trained physician, continued mass vaccination campaigns will enable new, more infectious viral variants.
Even if we had complete uptake in vaccines and complete masking, Malone said, CDC data makes it clear that at best we can slow the spread of Delta but we can’t stop it.
New CDC studies show waning vaccine immunity to Delta variant
Two studies released Aug. 24 by the CDC showed fully vaccinated Americans’ immunity to COVID is waning as the Delta variant now makes up 98.8% of U.S. COVID cases.
One study found vaccine effectiveness among frontline healthcare workers declined by nearly 30 percentage points since the Delta variant became the dominant strain in the U.S.
The analysis also concluded COVID vaccines were only 80% effective in preventing infection among the frontline healthcare workers.
The second study examined 43,000 Los Angeles residents 16 and older. Between May 1 and July 25, 25.3% of COVID infections occurred in fully vaccinated persons and 3.3% were in partially vaccinated persons.
The CDC cautioned in its report that vaccine effectiveness “might also be declining as time since vaccination increases and because of poor precision in estimates due to limited number of weeks of observation.”
The publication of the new studies followed a week after the CDC released its first three reports on vaccine efficacy — which also showed waning vaccine protection against the Delta variant.
172 days and counting, CDC ignores The Defender’s inquiries
According to the CDC website, “the CDC follows up on any report of death to request additional information and learn more about what occurred and to determine whether the death was a result of the vaccine or unrelated.”
On March 8, The Defender contacted the CDC with a written list of questions about reported deaths and injuries related to COVID vaccines. We have made repeated attempts, by phone and email, to obtain a response to our questions.
Despite multiple phone and email communications with many people at the CDC, and despite being told that our request was in the system and that someone would respond, we have not yet received answers to any of the questions we submitted. It has been 172 days since we sent our first email to the CDC requesting information.
Children’s Health Defense asks anyone who has experienced an adverse reaction, to any vaccine, to file a report following these three steps.
©August 2021 Children’s Health Defense, Inc. This work is reproduced and distributed with the permission of Children’s Health Defense, Inc. Want to learn more from Children’s Health Defense? Sign up for free news and updates from Robert F. Kennedy, Jr. and the Children’s Health Defense. Your donation will help to support us in our efforts.
Connect with Children’s Health Defense

Truth Comes to Light highlights writers and video creators who ask the difficult questions while sharing their unique insights and visions.
Everything posted on this site is done in the spirit of conversation. Please do your own research and trust yourself when reading and giving consideration to anything that appears here or anywhere else.